Abstract
Objective
To determine if racial differences are associated with Neonatal Opioid Withdrawal Syndrome (NOWS) severity.
Study design
A 10-year (2008–2017) retrospective cohort of infants ≥35 weeks gestation with prenatal exposure to opioids was included. The primary measure was the need for pharmacotherapy. Multivariable logistic regression and propensity score analysis were performed.
Results
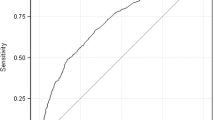
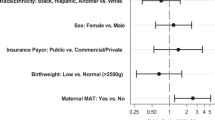
Among 345 infants with NOWS, 111 (32%) were black infants with 70% of them requiring pharmacotherapy as compared with 84% of white infants. Upon adjusting for significant covariates (methadone, benzodiazepine use, and gestational age), black infants were 57% less likely than whites to require pharmacotherapy (Odds ratio: 0.43, 95%CI: 0.22–0.80, p = 0.009). Similar results were observed with propensity score analysis.
Conclusions
Significant racial disparity observed may be secondary to genetic variations in opioid pharmacogenomics and/or extrinsic factors. Large-scale studies are warranted to include race in predictive models for early pharmacological intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kocherlakota P. Neonatal abstinence syndrome. Pediatrics. 2014;134:e547–61. https://doi.org/10.1542/peds.2013-3524
Patrick SW, Davis MM, Lehman CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009–2012. J Perinatol. 2015;35:650–5. https://doi.org/10.1038/jp.2015.36
Tolia V, Patrick S, Bennett M, Murthy K, Sousa J, Smith B. et al. Increasing Incidence of the neonatal abstinence syndrome in U.S neonatal ICUs. N Engl J Med. 2015;372:2118–26. https://doi.org/10.1056/NEJMsa1500439.
Berghella V, Lim PJ, Hill MK, Cherpes J, Chennat J, Kaltenbach K. Maternal methadone dose and neonatal withdrawal. Am J Obstet Gynecol. 2003;189:312–7.
Lewis T, Dinh J, Leeder JS. Genetic determinants of fetal opiate exposure and risk of neonatal abstinence syndrome: knowledge deficits and prospects for future research. Clin Pharmacol Ther. 2015;98:309–20.
MacMillan KDL, Rendon CP, Verma K, Riblet N, Washer DB, Volpe Holmes A. Association of rooming-in with outcomes for neonatal abstinence syndrome: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:345–51. https://doi.org/10.1001/jamapediatrics.2017.5195
McQueen K, Murphy-Oikonen J. Neonatal abstinence syndrome. N Engl J Med. 2016;375:2468–79. https://doi.org/10.1056/NEJMra1600879
Han LW, Gao C, Mao Q. An update on expression and function of P-gp/ABCB1 and BCRP/ABCG2 in the placenta and fetus. Expert Opin Drug Metabol Toxicol. 2018;14:817–29.
Hemauer SJ, Patrikeeva SL, Wang X, Abdelrahman D, Hankins G, Ahmed M, et al. Role of transporter-mediated efflux in the placental biodisposition of bupropion and its metabolite, OH-bupropion. Biochem Pharmacol. 2010;80:1080–6.
Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–99.
Kroetz DL, Pauli-Magnus C, Hodges LM, Huang CC, Kawamoto M, Johns SJ, et al. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharm Genom. 2003;13:481–94.
Lam J, Koren G. P-glycoprotein in the developing human brain: a review of the effects of ontogeny on the safety of opioids in neonates. Ther Drug Monit. 2014;36:699–705.
Leschziner G, Andrew T, Pirmohamed M, Johnson M. ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharm J 2007;7:154.
Wachman EM, Hayes MJ, Sherva R, Brown MS, Shrestha H, Logan BA, et al. Association of maternal and infant variants in PNOC and COMT genes with neonatal abstinence syndrome severity. Am J Addict. 2017;26:42–9. https://doi.org/10.1111/ajad.12483
Alexander MJ, Kiang MV, Barbieri M. Trends in Black and White Opioid Mortality in the United States, 1979-2015. Epidemiology. 2018;29:707–15.
Brown ES, Tirado C, Minhajuddin A, Hillhouse M, Adinoff B, Ling W, et al. Association of race and ethnicity with withdrawal symptoms, attrition, opioid use, and side-effects during buprenorphine therapy. J Ethn Subst Abus. 2010;9:106–14.
Fukuda T, Chidambaran V, Mizuno T, Venkatasubramanian R, Ngamprasertwong P, Olbrecht V, et al. OCT1 genetic variants influence the pharmacokinetics of morphine in children. Pharmacogenomics. 2013;14:1141–51.
Kosten, T, Rayford B. Effects of ethnicity on low-dose opiate stabilization. J Subst Abus Treat. 1995;12:111–6.
Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GD, Ahmed MS. Opiates inhibit paclitaxel uptake by P-glycoprotein in preparations of human placental inside-out vesicles. Biochem Pharmacol. 2009;78:1272–8.
Hoffmeyer S1, Burk O, von Richter O, Arnold HP, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 2000;97:3473–8.
Nielsen DA, Hamon S, Yuferov V, Jackson C, Ho A, Ott J, et al. Ethnic diversity of DNA methylation in the OPRM1 promoter region in lymphocytes of heroin addicts. Hum Genet. 2010;127:639–49.
Nielsen DA, Utrankar A, Reyes JA, Simons DD, Kosten TR. Epigenetics of drug abuse: Predisposition or response. Pharmacogenomics. 2012;13:1149–60.
Nielsen DA, Yuferov V, Hamon S, Jackson C, Ho A, Ott J, et al. Increased OPRM1 DNA methylation in lymphocytes of methadone-maintained former heroin addicts. Neuropsychopharmacology. 2009;34:867.
Wachman E, Hayes M, Brown M, Paul J, Harvey-Wilkes K, Terrin N, et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. J Am Med Assoc. 2013;309:1821–7. https://doi.org/10.1001/jama.2013.3411.
Wachman EM, Hayes MJ, Lester BM, Terrin N, Brown MS, Nielsen DA, et al. Epigenetic variation in the mu-opioid receptor gene in infants with neonatal abstinence syndrome. J Pediatr. 2014;165:472–8. https://doi.org/10.1016/j.jpeds.2014.05.040
Wachman EM, Hayes MJ, Sherva R, Brown MS, Davis JM, Farrer LA, et al. Variations in opioid receptor genes in neonatal abstinence syndrome. Drug Alcohol Depend. 2015;155:253–9. https://doi.org/10.1016/j.drugalcdep.2015.07.001
Cole FS, Wegner DJ, Davis JM. The genomics of neonatal abstinence syndrome. Front Pediatr. 2017;5:176. https://doi.org/10.3389/fped.2017.00176
Crist RC, Berrettini WH. Pharmacogenetics of OPRM1. Pharmacol Biochem Behav. 2014;123:25–33.
Doehring A, Oertel BG, Sittl R, Lötsch J. Chronic opioid use is associated with increased DNA methylation correlating with increased clinical pain. PAIN®. 2013;154:15–23.
Reddy UM, Davis JM, Ren Z, Greene MF. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American Congress of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstet Gynecol. 2017;130:10.
Ruano G, Kost JA. Fundamental considerations for genetically-guided pain management with opioids based on CYP2D6 and OPRM1 polymorphisms. Pain Physician. 2018;21:E611–21.
Bagley SM, Wachman EM, Holland E, Brogly SB. Review of the assessment and management of neonatal abstinence syndrome. Addict Sci Clin Pract. 2014;9:19. https://doi.org/10.1186/1940-0640-9-19
Austin Peter C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424.
Seunghee B, Ho PS, Eugene W, Rang PY, Jung KH. Propensity score matching: a conceptual review for radiology researchers. Korean J Radiol. 2015;16:286.
O’Connor AB, O’Brien L, Alto WA. Are there gender related differences in neonatal abstinence syndrome following exposure to buprenorphine during pregnancy? J Perinat Med. 2013;41:621–3.
Chorbov VM, Todorov AA, Lynskey MT, Cicero TJ. Elevated levels of DNA methylation at the OPRM1 promoter in blood and sperm from male opioid addicts. J Opioid Manag. 2011;7:258–64.
Pauli-Magnus C, Kroetz DL. Functional implications of genetic polymorphisms in the multidrug resistance gene MDR1 (ABCB1). Pharm Res. 2004;21:904–13.
Mistry CJ, Bawor M, Desai D, Marsh DC, Samaan Z. Genetics of Opioid Dependence: A Review of the Genetic Contribution to Opioid Dependence. Curr Psychiatry Rev. 2014;10:156–67.
Johnson K, Greenough A, Gerada C. Maternal drug use and length of neonatal unit stay. Addiction. 2003;98:785–9.
Seligman NS, Salva N, Hayes EJ, Dysart KC, Pequignot EC, Baxter JK. Predicting length of treatment for neonatal abstinence syndrome in methadone-exposed neonates. Am J Obstet Gynecol. 2008;199:396–e1.
Sun M, Kingdom J, Baczyk D, Lye S, Matthews S, Gibb W. Expression of the multidrug resistance P-glycoprotein (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta 2006;27:602–9.
Liebling EJ, Yedinak JL, Green TC, Hadland SE, Clark MA, Marshall BD. Access to substance use treatment among young adults who use prescription opioids non-medically. Subst Abus Treat Prev Policy. 2016;11:38.
Mathias AA, Hitti J, Unadkat JD. P-glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages. Am J Physiol Regul Integr Comp Physiol. 2005;289:R963–9.
Short VL, Hand DJ, MacAfee L, Abatemarco DJ, Terplan M. Trends and disparities in receipt of pharmacotherapy among pregnant women in publically funded treatment programs for opioid use disorder in the United States. J Subst Abus Treat. 2018;89:67–74.
Acknowledgements
We would like to thank our multidisciplinary team at the University of Maryland Medical center, including nurses, nurse practitioners, and physicians who are constantly involved in taking care of these patients. We would also like to thank Ms. Kelly Tracey in helping us identify patients for this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parikh, A., Gopalakrishnan, M., Azeem, A. et al. Racial association and pharmacotherapy in neonatal opioid withdrawal syndrome. J Perinatol 39, 1370–1376 (2019). https://doi.org/10.1038/s41372-019-0440-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0440-8
This article is cited by
-
Polygenic risk scores and the need for pharmacotherapy in neonatal abstinence syndrome
Pediatric Research (2023)
-
Implicit Racial Bias in Evaluation of Neonatal Opiate Withdrawal Syndrome
Journal of Racial and Ethnic Health Disparities (2023)
-
The Influence of Mediators on the Relationship Between Antenatal Opioid Agonist Exposure and the Severity of Neonatal Opioid Withdrawal Syndrome
Maternal and Child Health Journal (2023)
-
Predictors of pharmacologic therapy for neonatal opioid withdrawal syndrome: a retrospective analysis of a statewide database
Journal of Perinatology (2021)
-
Racial differences in opioid withdrawal syndrome among neonates with intrauterine opioid exposure
Pediatric Research (2021)