Abstract
Objective
To compare neurodevelopmental and visual outcomes in preterm infants treated with intravitreal bevacizumab (IVB) to laser ablation at 18–24 months corrected age.
Study design
A retrospective study was performed. The primary outcome was neurodevelopmental impairment (NDI). Secondary neurodevelopmental outcomes were significant NDI (sNDI), cerebral palsy, hearing loss, and composite scores of the Bayley Scales of Infant Development, Third edition. Visual outcomes included structural and refractive outcomes. Adjusted odds ratios (AOR) were calculated controlling for GA, sex, and ROP severity and confounding baseline characteristics using a cutoff of p < 0.20.
Results
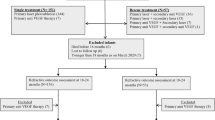
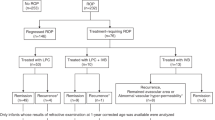
Thirty-four (60 eyes) infants receiving IVB and 30 (51 eyes) laser were included. No significant differences were identified in NDI (AOR 1.77, 95% CI 0.46, 6.73) or sNDI (AOR 2.31, 95% CI 0.75, 7.14). There were no other differences in outcomes.
Conclusions
Larger randomized trials are required to establish long-term efficacy and safety of IVB in preterm neonates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94.
Smith LE. Through the eyes of a child: understanding retinopathy through ROP the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2008;49:5177–82.
Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–15.
Isaac M, Mireskandari K, Tehrani N. Type I ROP, treatment with bevacizumab versus laser: comparison of visual function, structural outcome and frequency of follow-up. J Am Assoc Pediatr Ophthalmol Strabismus. 2014;18:e5.
Lien R, Yu MH, Hsu KH, Liao PJ, Chen YP, Lai CC, et al. Neurodevelopmental outcomes in infants with retinopathy of prematurity and bevacizumab treatment. PLoS ONE. 2016;11:e0148019.
Morin J, Luu TM, Superstein R, Ospina LH, Lefebvre F, Simard MN, et al. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics. 2016;137:e20153218.
Martinez-Castellanos MA, Schwartz S, Hernandez-Rojas ML, Kon-Jara VA, Garcia-Aguirre G, Guerrero-Naranjo JL, et al. Long-term effect of antiangiogenic therapy for retinopathy of prematurity up to 5 years of follow-up. Retina. 2013;33:329–38.
Wu WC, Lien R, Liao PJ, Wang NK, Chen YP, Chao AN, et al. Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol. 2015;133:391–7.
Network TCN. The Canadian Neonatal Network Home Page. 2009 Available from: http://www.canadianneonatalnetwork.org/portal/ [cited 16 May 2018].
Network TCNF-u. The Canadian Neonatal Follow-up Network Home Page. 2009. Available from: http://www.cnfun.ca/ [cited 16 May 2018].
Rezai KA, Eliott D, Ferrone PJ, Kim RW. Near confluent laser photocoagulation for the treatment of threshold retinopathy of prematurity. Arch Ophthalmol. 2005;123:621–6.
Fallaha N, Lynn MJ, Aaberg TM Jr., Lambert SR. Clinical outcome of confluent laser photoablation for retinopathy of prematurity. J Aapos. 2002;6:81–85.
Network TCN. CNN Abstractor’s Manual v. 1.3.4. Toronto, Ontario: Canadian Neonatal Network; 2011.
Bassler D, Stoll BJ, Schmidt B, Asztalos EV, Roberts RS, Robertson CM, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123:313–8.
Schlapbach LJ, Adams M, Proietti E, Aebischer M, Grunt S, Borradori-Tolsa C, et al. Outcome at two years of age in a Swiss national cohort of extremely preterm infants born between 2000 and 2008. BMC Pediatr. 2012;12:198.
Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. Intensive care for extreme prematurity–moving beyond gestational age. N Engl J Med. 2008;358:1672–81.
Amer R, Moddemann D, Seshia M, Alvaro R, Synnes A, Lee KS, et al. Neurodevelopmental outcomes of infants born at <29 weeks of gestation admitted to canadian neonatal intensive care units based on location of birth. J Pediatr. 2018;196:31.e31–37.e31.
Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–61.
Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanic K, et al. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–9.
Ing C, DiMaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, et al. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–85.
Bayley N. Bayley Scales of Infant and Toddler Development, 3rd edn. San Antonio, TX: Psychocorp; 2005.
Squires J, Bricker D. Ages & Stages Questionnaires®, Third Edition (ASQ- 3™). A parent-completed child-monitoring system. Baltimore, MD: Paul H. Brookes Publishing Co; 2009.
Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med child Neurol. 1997;39:214–23.
Sharp M, DeMauro SB. Counterbalanced comparison of the BSID-II and Bayley-III at eighteen to twenty-two months corrected age. J Dev Behav Pediatr. 2017;38:322–9.
Schonhaut L, Armijo I, Schönstedt M, Alvarez J, Cordero M. Validity of the ages and stages questionnaires in term and preterm infants. Pediatrics. 2013;131:e1468–74.
Lowe JR, Erickson SJ, Schrader R, Duncan AF. Comparison of the Bayley II Mental Developmental Index and the Bayley III Cognitive Scale: are we measuring the same thing? Acta Paediatr. 2012;101:e55–8.
Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy. Dev Med Child Neurol Suppl. 2007;109:8–14.
Weisz DE, Mirea L, Rosenberg E, Jang M, Ly L, Church PT, et al. Association of patent ductus arteriosus ligation with death or neurodevelopmental impairment among extremely preterm infants. JAMA Pediatr. 2017;171:443–9.
Glass TJA, Chau V, Gardiner J, Foong J, Vinall J, Zwicker JG, et al. Severe retinopathy of prematurity predicts delayed white matter maturation and poorer neurodevelopment. Arch Dis Child Fetal Neonatal Ed. 2017;102:F532–7.
Kennedy KA, Mintz-Hittner HA, Group B-RC. Medical and developmental outcomes of bevacizumab versus laser for retinopathy of prematurity. J AAPOS. 2018;22:61.e1–5.e1.
Chen TA, Schachar IH, Moshfeghi DM. Outcomes of intravitreal bevacizumab and diode laser photocoagulation for treatment-warranted retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retin. 2018;49:126–31.
Haigh JJ. Role of VEGF in organogenesis. Organogenesis. 2008;4:247–56.
Khalili S, Shifrin Y, Pan J, Belik J, Mireskandari K. The effect of a single anti-vascular endothelial growth factor injection on neonatal growth and organ development: in-vivo study. Exp Eye Res. 2018;169:54–59.
Jo DH, Park SW, Cho CS, Powner MB, Kim JH, Fruttiger M. Intravitreally injected anti-VEGF antibody reduces brown fat in neonatal mice. PLoS ONE. 2015;10:e0134308.
Chen YH, Chen SN, Lien RI, Shih CP, Chao AN, Chen KJ, et al. Refractive errors after the use of bevacizumab for the treatment of retinopathy of prematurity: 2-year outcomes. Eye (Lond). 2014;28:1080–6.
Geloneck MM, Chuang AZ, Clark WL, Hunt MG, Norman AA, Packwood EA, et al. Refractive outcomes at age 2½ years in the bevacizumab eliminates the angiogenic threat for retinopathy of prematurity trial: a randomized clinical trial. J Am Assoc Pediatr Ophthalmol Strabismus. 2013;17:e1–7.
Sankar MJ, Sankar J, Chandra P. Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity. Cochrane Database Syst Rev. 2018;1:CD009734.
VanderVeen DK, Melia M, Yang MB, Hutchinson AK, Wilson LB, Lambert SR. Anti-vascular endothelial growth factor therapy for primary treatment of type 1 retinopathy of prematurity: a report by the American Academy of Ophthalmology. Ophthalmology. 2017;124:619–33.
Zhang G, Yang M, Zeng J, Vakros G, Su K, Chen M, et al. Comparison of intravitreal injection of ranibizumab versus laser therapy for zone II treatment-requiring retinopathy of prematurity. Retina. 2017;37:710–7.
Lepore D, Quinn GE, Molle F, Orazi L, Baldascino A, Ji MH, et al. Follow-up to age 4 years of treatment of type 1 retinopathy of prematurity intravitreal bevacizumab injection versus laser: fluorescein angiographic findings. Ophthalmology. 2018;125:218–26.
Acknowledgements
The authors gratefully acknowledge all site investigators of the Canadian Neonatal Network (CNN) and Canadian Neonatal Follow-Up Network (CNFUN). We would also like to extend our thanks to the data abstractors of the CNN and CNFUN at three participating hospitals, as well as the staff at the Maternal-Infant Care Research Centre at Mount Sinai Hospital, Toronto, ON for providing organizational support for this project.
Funding source
Although no specific funding has been received for this study, organizational support for the Canadian Neonatal Network was provided by the Maternal-Infant Care Research Centre (MiCare) at Mount Sinai Hospital in Toronto. MiCare and the Canadian Neonatal Follow-Up Network are supported by a Canadian Institutes of Health Research (CIHR) Team Grant (FRN87518) and in-kind support from Mount Sinai Hospital. PSS holds an Applied Research Chair in Reproductive and Child Health Services and Policy Research awarded by the CIHR (APR-126340). The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
MI and KR contributed to conceptualizing the study and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted. JY carried out the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted. AAA contributed to data collected, aided in formulating the study proposal, reviewed and revised the manuscript, and approved the final manuscript as submitted. KM contributed to the concept, design and interpretation of data, critically reviewed and revised the draft manuscript for intellectual content, and approved the final submitted version of the article. LGL, EK, and RB oversaw and interpreted all the developmental assessments at their respective institutions, reviewed and critically revised the manuscript, and approved the final manuscript as submitted. PSS reviewed the initial study proposal and research ethics application, contributed to the concept of the study, acquisition, analysis and interpretation of data, reviewed and critically revised the manuscript, and approved the final manuscript as submitted. NT proposed the research question, conceptualized the study, reviewed the initial study proposal and research ethics application, contributed to the acquisition, analysis and interpretation of data, reviewed and critically revised the manuscript, and approved the final manuscript as submitted. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raghuram, K., Isaac, M., Yang, J. et al. Neurodevelopmental outcomes in infants treated with intravitreal bevacizumab versus laser. J Perinatol 39, 1300–1308 (2019). https://doi.org/10.1038/s41372-019-0420-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0420-z
This article is cited by
-
Association of low hemoglobin at birth and neurodevelopmental outcomes in preterm neonates ≤28 weeks’ gestation: a retrospective cohort study
Journal of Perinatology (2024)
-
Pulmonary function in school-age children following intravitreal injection of bevacizumab for retinopathy of prematurity
Scientific Reports (2022)
-
Comparison of adverse events between intravitreal anti-VEGF and laser photocoagulation for treatment-requiring retinopathy of prematurity: a systematic review
International Ophthalmology (2022)
-
Neurodevelopmental outcomes following bevacizumab treatment for retinopathy of prematurity: a systematic review and meta-analysis
Journal of Perinatology (2021)
-
Neurodevelopmental outcomes following bevacizumab treatment for retinopathy of prematurity: a systematic review and meta-analysis
Journal of Perinatology (2021)