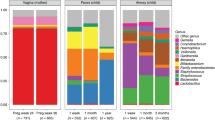
Abstract
Objective:
To determine the implications of supplemental vitamin C for pregnant tobacco smokers and its effects on the prevalence of pediatric asthma, asthma-related mortality, and associated costs.
Study design:
A decision-analytic model built via TreeAge compared the outcome of asthma in a theoretical annual cohort of 480,000 children born to pregnant smokers through 18 years of life. Vitamin C supplementation (500 mg/day) with a standard prenatal vitamin was compared to a prenatal vitamin (60 mg/day). Model inputs were derived from the literature. Deterministic and probabilistic sensitivity analyses assessed the impact of assumptions.
Result:
Additional vitamin C during pregnancy would prevent 1637 cases of asthma at the age of 18 per birth cohort of pregnant smokers. Vitamin C would reduce asthma-related childhood deaths and save $31,420,800 in societal costs over 18 years per birth cohort.
Conclusion:
Vitamin C supplementation in pregnant smokers is a safe and inexpensive intervention that may reduce the economic burden of pediatric asthma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tong VT, Dietz PM, Morrow B, D’Angelo DV, Farr SL, Rockhill KM, et al. Trends in smoking before, during, and after pregnancy – Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000-2010. MMWR. 2013;62(SS06):1–19.
Centers for Disease Control and Prevention. Tobacco use and pregnancy. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/tobaccousepregnancy/. Accessed 25 July 2016.
Hayatbakhsh MR, Sadasivam S, Mamun AA, Najman JM, Williams GM, O’Callaghan MJ. Maternal smoking during and after pregnancy and lung function in early adulthood: a prospective study. Thorax. 2009;64:810–4.
Maritz GS, Rayise SS. Effect of maternal nicotine exposure on neonatal rat lung development: protective effect of maternal ascorbic acid supplementation. Exp Lung Res. 2011;37:57–65.
Proskocil BJ, Sekhon HS, Clark JA, Lupo SL, Jia Y, Hull WM, et al. Vitamin C prevents the effects of prenatal nicotine on pulmonary function in newborn monkeys. Am J Respir Crit Care Med. 2005;171:1032–9.
Håland G, Lødrup Carlsen KC, Sandvik L, Devulapalli CS, Munthe-Kaas MC, Pettersen M, et al. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–9.
Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–64.
Centers for Disease Control and Prevention. Summary health statistics tables for U.S. children: national health interview survey, 2015. https://www.cdc.gov/nchs/fastats/asthma.htm. Accessed 30 August 2017.
Akuete K, Oh SS, Thyne S, Rodriguez-Santana JR, Chapela R, Meade K, et al. Ethnic variability in persistent asthma after in utero tobacco exposure. Pediatrics. 2011;128:e623–30.
Bui AL, Dieleman JL, Hamavid H, Birger M, Chapin A, Duber HC, et al. Spending on children’s personal health care in the United States, 1996-2013. JAMA Pediatr. 2017;171:181–9.
Taylor B, Wadsworth J. Maternal smoking during pregnancy and lower respiratory tract illness in early life. Arch Dis Child. 1987;62:786–91.
Lozano P, Sullivan SD, Smith DH, Weiss KB. The economic burden of asthma in US children: estimates from the National Medical Expenditure Survey. J Allergy Clin Immunol. 1999;104:957–63.
Wang LY, Zhong Y, Wheeler L. Direct and indirect costs of asthma in school-age children. Prev Chronic Dis. 2005;2:1–10.
Lo JO, Schabel MC, Roberts VHJ, Morgan TK, Rasanen JP, Kroenke CD, et al. Vitamin C supplementation ameliorates the adverse effects of nicotine on placental hemodynamics and histology in non-human primates. Am J Obstet Gynecol. 2015;212:370e1–8.
Astuti Y, Wardhana A, Watkins J, Wulaningsih W. Cigarette smoking and telomere length: a systematic review of 84 studies and meta-analysis. Environ Res. 2017;158:480–9.
Ip P, Chung BH, Ho FK, Chan GC, Deng W, Wong WH, et al. Prenatal tobacco exposure shortens telomere length in children. Nicotine Tob Res. 2017;19:111–8.
Furumoto K, Inoue E, Nagao N, Hiyama E, Miwa N. Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sci. 1998;63:935–48.
McEvoy CT, Schilling D, Clay N, Jackson K, Go MD, Spitale P, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants. JAMA. 2014;311:2074–82.
Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II – an ISPOR good research practices task force report. Value Health. 2015;18:161–72.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–103.
Martinez FD, Morgan WJ, Wright AL, Holberg C, Taussig LM, Group Health Medical Associates. . Initial airway function is a risk factor for recurrent wheezing respiratory illnesses during the first three years of life. Am Rev Respir Dis. 1991;143:312–6.
Lødrup Carlsen KC, Jaakkola JJK, Nafstad P, Carlsen K-H. In utero exposure to cigarette smoking influences lung function at birth. Eur Respir J. 1997;10:1774–9.
Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–22.
Hovland V, Riiser A, Mowinckel P, Carlsen K-H, Lødrup Carlsen KC. The significance of early recurrent wheeze for asthma outcomes in late childhood. Eur Resp J. 2013;41:838–45.
Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Mathews TJ. Births: final data for 2015. Natl Vital Stat Rep. 2017;66:1–69.
Chiou C, Weaver MR, Bell MA, Lee TA, Krieger JW. Development of the multi-attribute pediatric asthma health outcome measure (PAHOM). Int J Qual Health Care. 2005;17:23–30.
U.S. Bureau of Labor Statistics. Consumer price index for all urban consumers: medical care (CPIMEDSL). https://www.bls.gov/cpi/. Accessed 15 August 2016.
Kurukulaaratchy RJ, Fenn MH, Waterhouse LM, Matthews SM, Holgate ST, Arshad SH. Characterization of wheezing phenotypes in the first 10 years of life. Clin Exp Allergy. 2003;33:573–8.
Martinez FD. What have we learned from the Tuscon Children’s Respiratory Study? Paediatr Respir Rev. 2002;3:193–7.
Piippo-Savolainen E, Remes S, Kannisto S, Korhonen K, Korppi M. Asthma and lung function 20 years after wheezing in infancy: results from a prospective follow-up study. Arch Pediatr Adolesc Med. 2004;158:1070–6.
Schmidt CW. Growing a new study: environmental influences on child health outcomes. Environ Health Perspect. 2015;123:A260–3.
Russell T, Crawford M, Woodby L. Measurements for active cigarette smoke exposure in prevalence and cessation studies: why simply asking pregnant women isn’t enough. Nicotine Tob Res. 2004;6 Suppl 2:S141–51.
Porter L, Duke J, Hennon M, Dekevich D, Crankshaw E, Homsi G, et al. Electronic cigarette and traditional cigarette use among middle and high school students in Florida, 2011-2014. PLoS ONE. 2015;10:e0124385.
Ion R, Bernal AL. Smoking and preterm birth. Reprod Sci. 2015;22:918–26.
Filion KB, Abenhaim HA, Mottillo S, Joseph L, Gervais A, O’Loughlin J, et al. The effect of smoking cessation counselling in pregnant women: a meta-analysis of randomised controlled trials. BJOG. 2011;118:1422–8.
Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;9:CD010078.
Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA. 2016;315:362–70.
Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Schoos AM, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530–9.
Acknowledgements
Cindy T. McEvoy and Kelvin D. MacDonald are supported by the NIH, National Heart Lung Blood Institute, R01 HL105447 with co-funding from the Office of Dietary Supplement, UG3OD023288; R01H L129060.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yieh, L., McEvoy, C.T., Hoffman, S.W. et al. Cost effectiveness of vitamin c supplementation for pregnant smokers to improve offspring lung function at birth and reduce childhood wheeze/asthma. J Perinatol 38, 820–827 (2018). https://doi.org/10.1038/s41372-018-0135-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-018-0135-6
This article is cited by
-
A meta-analysis of the effects of vitamin C supplementation for pregnant smokers on the pulmonary function of their offspring
BMC Pregnancy and Childbirth (2024)
-
The effects of vitamin C on respiratory, allergic and immunological diseases: an experimental and clinical-based review
Inflammopharmacology (2023)
-
Improving fetal lung development with vitamin C and reducing asthma in children
Journal of Perinatology (2018)