Abstract
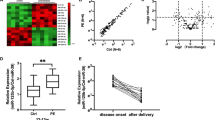
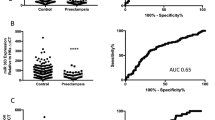
This study explored the correlation of MMP-9 with miR-181a-5p in severe preeclampsia (SPE). Placental tissues and serum were collected from 30 pregnant SPE patients (aged 29.42 ± 3.75) and 30 normal pregnant women (aged 27.72 ± 2.21), followed by detecting MMP-9 and miR-181a-5p fold changes using RT-qPCR, and grouped as follows: high expression groups (≥median value): H-MMP-9 and H-miR-181a-5p vs. low expression groups (<median value): L-MMP-9 and L-miR-181a-5p. MMP-9 was weakly expressed (F = 709.99, p < 0.001; F = 670.45, p < 0.001) (serum: 0.41 ± 0.06 (fold changes); placenta: 0.42 ± 0.09 (fold changes)), whereas miR-181a-5p was highly expressed (F = 284.93, p < 0.001; F = 353.78, p < 0.001) (serum: 2.26 ± 0.39; placenta: 2.02 ± 0.29) in SPE patients. MMP-9 was negatively correlated with miR-181a-5p (serum: r = −0.5767, p = 0.0009; placenta: r = −0.5667, p = 0.0011) in SPE patients. MMP-9 showed positive-correlation with gestational week at delivery (r = 0.7625; p < 0.0001) and infant weight (r = 0.4947; p < 0.0001) of SPE patients. miR-181-5p showed negative-correlation (gestational week at delivery: r = −0.5614, p = 0.0012; infant weight: r = −0.4081, p = 0.0252). H-MMP-9 group had lower adverse outcome than L-MMP-9 group (p = 0.0006), and H-miR-181a-5p group had higher adverse outcome than L-miR-181a-5p group (p = 0.0036). In brief, MMP-9 was negatively correlated with miR-181a-5p in serum and placenta of SPE patients. MMP-9 and miR-181a-5p affected gestational week at delivery and infant weight, providing novel targets for SPE treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data and materials availability
All the data generated or analyzed during this study are included in this published article.
References
Pankiewicz K, Fijalkowska A, Issat T, Maciejewski TM. Insight into the key points of preeclampsia pathophysiology: uterine artery remodeling and the role of MicroRNAs. Int J Mol Sci. 2021;22:3132.
Vigil-De Gracia P, Ludmir J. The use of magnesium sulfate for women with severe preeclampsia or eclampsia diagnosed during the postpartum period. J Matern Fetal Neonatal Med. 2015;28:2207–9.
Ray A, Ray S. Epidural therapy for the treatment of severe pre-eclampsia in non labouring women. Cochrane Database Syst Rev. 2017;11:CD009540.
Arulkumaran N, Lightstone L. Severe pre-eclampsia and hypertensive crises. Best Pract Res Clin Obstet Gynaecol. 2013;27:877–84.
Ferreira I, Peeters LL, Stehouwer CD. Preeclampsia and increased blood pressure in the offspring: meta-analysis and critical review of the evidence. J Hypertens. 2009;27:1955–9.
Pasaribu HP, Hariman H, Roeshadi RH, Koh SC. Soluble vascular cell adhesion molecule-1 and magnesium sulfate with nifedipine treatment in Indonesian women with severe pre-eclampsia. Inter Med Appl Sci. 2016;8:97–102.
Shen M, Smith GN, Rodger M, White RR, Walker MC, Wen SW. Comparison of risk factors and outcomes of gestational hypertension and pre-eclampsia. PLoS One. 2017;12:e0175914.
WHO recommendations: Policy of interventionist versus expectant management of severe pre-eclampsia before term: Geneva, 2018.
Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141:1202–7.
Skalis G, Katsi V, Miliou A, Georgiopoulos G, Papazachou O, Vamvakou G, et al. MicroRNAs in preeclampsia. Microrna. 2019;8:28–35.
Lv Y, Lu C, Ji X, Miao Z, Long W, Ding H, et al. Roles of microRNAs in preeclampsia. J Cell Physiol. 2019;234:1052–61.
Gusar V, Timofeeva A, Chagovets V, Kan N, Vasilchenko O, Prozorovskaya K et al. Preeclampsia: the interplay between oxygen-sensitive mirnas and erythropoietin. J Clin Med 2020;9:574.
Huang X, Wu L, Zhang G, Tang R, Zhou X. Elevated MicroRNA-181a-5p contributes to trophoblast dysfunction and preeclampsia. Reprod Sci. 2019;26:1121–9.
Chen J, Khalil RA. Matrix metalloproteinases in normal pregnancy and preeclampsia. Prog Mol Biol Transl Sci. 2017;148:87–165.
Espino YSS, Flores-Pliego A, Espejel-Nunez A, Medina-Bastidas D, Vadillo-Ortega F, Zaga-Clavellina V et al. New insights into the role of matrix metalloproteinases in preeclampsia. Int J Mol Sci 2017;18:1448.
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43.
Gottardi E, Lecarpentier E, Villette C, Berman A, Redel D, Tsatsaris V, et al. Preeclampsia before 26 weeks of gestation: obstetrical prognosis for the subsequent pregnancy. J Gynecol Obstet Hum Reprod. 2021;50:102000.
Takahashi N, Li F, Fushima T, Oyanagi G, Sato E, Oe Y, et al. Vitamin B3 nicotinamide: a promising candidate for treating preeclampsia and improving fetal growth. Tohoku J Exp Med. 2018;244:243–8.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Abraham C, Kusheleva N. Management of pre-eclampsia and eclampsia: a simulation. MedEdPORTAL. 2019;15:10832.
Vashukova ES, Glotov AS, Fedotov PV, Efimova OA, Pakin VS, Mozgovaya EV, et al. Placental microRNA expression in pregnancies complicated by superimposed preeclampsia on chronic hypertension. Mol Med Rep. 2016;14:22–32.
Feng H, Wang L, Zhang M, Zhang Z, Guo W, Wang X. Ratio of matrix metalloproteinase-2 to -9 is a more accurate predictive biomarker in women with suspected pre-eclampsia. Biosci Rep. 2017;37:BSR20160508.
Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145:1–33. Suppl 1
Nascimento RA, Possomato-Vieira JS, Bonacio GF, Rizzi E, Dias-Junior CA. Reductions of circulating nitric oxide are followed by hypertension during pregnancy and increased activity of matrix metalloproteinases-2 and -9 in rats. Cells 2019;8:1402.
Laskowska M. Altered maternal serum matrix metalloproteinases MMP-2, MMP-3, MMP-9, and MMP-13 in severe early- and late-onset preeclampsia. Biomed Res Int. 2017;2017:6432426.
Zhu A, Chu L, Ma Q, Li Y. Long non-coding RNA H19 down-regulates miR-181a to facilitate endothelial angiogenic function. Artif Cells Nanomed Biotechnol. 2019;47:2698–705.
Basu J, Agamasu E, Bendek B, Salafia CM, Mishra A, Lopez JV, et al. Correlation between placental matrix metalloproteinase 9 and tumor necrosis factor-alpha protein expression throughout gestation in normal human pregnancy. Reprod Sci. 2018;25:621–7.
Johannsson E, Henriksen T, Iversen PO. Increase in matrix metalloproteinases from endothelial cells exposed to umbilical cord plasma from high birth weight newborns. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1563–8.
Hromadnikova I, Kotlabova K, Krofta L A. History of preterm delivery is associated with aberrant postpartal MicroRNA expression profiles in mothers with an absence of other pregnancy-related complications. Int J Mol Sci 2021;22:4033.
Poon LC, Nekrasova E, Anastassopoulos P, Livanos P, Nicolaides KH. First-trimester maternal serum matrix metalloproteinase-9 (MMP-9) and adverse pregnancy outcome. Prenat Diagn. 2009;29:553–9.
Yao Y, Robinson AM, Zucchi FC, Robbins JC, Babenko O, Kovalchuk O, et al. Ancestral exposure to stress epigenetically programs preterm birth risk and adverse maternal and newborn outcomes. BMC Med. 2014;12:121.
Nissi R, Talvensaari-Mattila A, Kotila V, Niinimaki M, Jarvela I, Turpeenniemi-Hujanen T. Circulating matrix metalloproteinase MMP-9 and MMP-2/TIMP-2 complex are associated with spontaneous early pregnancy failure. Reprod Biol Endocrinol. 2013;11:2.
Hromadnikova I, Kotlabova K, Dvorakova L, Krofta L. Postpartum profiling of microRNAs involved in pathogenesis of cardiovascular/cerebrovascular diseases in women exposed to pregnancy-related complications. Int J Cardiol. 2019;291:158–67.
Author information
Authors and Affiliations
Contributions
Y.J.Z., L.W. contributed to the study concepts, study design, and definition of intellectual content; F.F.N. contributed to the literature research; Y.J.Z., L.W., F.F.N., Y.Y.L. contributed to the manuscript preparation and L.F. contributed to the manuscript editing and review; Y.J.Z., L.W., F.F.N. contributed to the experimental studies and data acquisition; Y.Y.L., L.F. contributed to the data analysis and statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study strictly followed the Declaration of Helsinki and was approved by the Ethics Committee of Shengli Oilfield Central Hospital. All subjects signed informed consent forms before participating in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, Y., Wang, L., Niu, F. et al. MMP-9 and miR-181a-5p in serum and placenta are associated with adverse outcomes of patients with severe preeclampsia and their infants. J Hum Hypertens 36, 1072–1077 (2022). https://doi.org/10.1038/s41371-021-00643-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-021-00643-z