Abstract
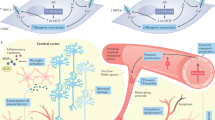
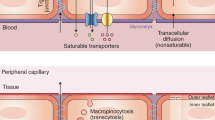
The blood–brain barrier (BBB) constitutes the complex anatomic and physiologic interface between the intravascular compartment and the central nervous system, and its integrity is paramount for the maintenance of the very sensitive homeostasis of the central nervous system. Arterial hypertension is a leading cause of morbidity and mortality. The BBB has been shown to be disrupted in essential hypertension. BBB integrity is important for central autonomic control and this may be implicated in the pathophysiology of hypertension. On the other hand, evidence from experimental studies indicates that BBB disruption can be present in both hypertensive disease and dementia syndromes, suggesting a possibly key position of loss of BBB integrity in the pathophysiological pathways linking arterial hypertension with cognitive decline. Although much still remains to be elucidated with respect to the exact underlying mechanisms, the discovery of novel pathological pathways has changed our understanding of adult dementia and central nervous system disease overall, pointing out—in parallel—new potential therapeutic targets. The aim of this review is to summarize current scientific knowledge relevant to the pathophysiologic pathways that are involved in the disruption of the BBB function and potentially mediate hypertension-induced cognitive impairment. In parallel, we underline the differential cognition-preserving effect of several antihypertensive agents of similar blood pressure-lowering capacity, highlighting the presence of previously under-recognized BBB-protective actions of these drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Mohammadi MT, Dehghani GA. Acute hypertension induces brain injury and blood–brain barrier disruption through reduction of claudins mRNA expression in rat. Pathol Res Pr. 2014;210:985–89.
Kalaria RN. Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr Rev. 2010;6:S74–S87.
Keller A. Breaking and building the wall: the biology of the blood–brain barrier in health and disease. Swiss Med Wkly. 2013;143:w13892.
Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood–brain barrier. Nat Med. 2013;19:1584–96.
Serlin Y, Shelef I, Knyazer B, Friedman A. Anatomy and physiology of the blood–brain barrier. Semin Cell Dev Biol. 2015;38:2–6.
Smith PM, Ferguson AV. Circulating signals as critical regulators of autonomic state–central roles for the subfornical organ. Am J Physiol Regul Integr Comp Physiol. 2010;299:R405–15.
Waki H, Gouraud SS, Maeda M, Raizada MK, Paton JF. Contributions of vascular inflammation in the brainstem for neurogenic hypertension. Respir Physiol Neurobiol. 2011;178:422–8.
Ronaldson PT, Davis TP. Targeting transporters: promoting blood–brain barrier repair in response to oxidative stress injury. Brain Res. 2015;1623:39–52.
Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304:H1598–H1614.
Lippoldt A, Kniesel U, Liebner S, Kalbacher H, Kirsch T, Wolburg H, et al. Structural alterations of tight junctions are associated with loss of polarity instroke-prone spontaneously hypertensive rat blood–brain barrier endothelial cells. Brain Res. 2000;885:251–61.
Tan KH, Harrington S, Purcell WM, Hurst RD. Peroxynitrite mediatesnitric oxide-induced blood–brain barrier damage. Neurochem Res. 2004;29:579–87.
Sparks DL, Scheff SW, Liu H, Landers TM, Coyne CM, Hunsaker JC 3rd. Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. J Neurol Sci. 1995;131:162–9.
Witt KA, Mark KS, Hom S, Davis TP. Effects of hypoxia-reoxygenation on rat blood–brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2003;285:2820–31.
Szarka N, Toth L, Czigler A, Kellermayer Z, Ungvari Z, Amrein K, et al. Single mild traumatic brain injury induces persistent disruption of the blood–brain barrier, neuroinflammation and cognitive decline in hypertensive rats. Int J Mol Sci. 2019;20:3223.
Parathath SR, Parathath S, Tsirka SE. Nitric oxide mediates neurodegeneration and breakdown of the blood–brain barrier in tPA-dependent excitotoxic injury in mice. J Cell Sci. 2006;119:339–49.
Enciu AM, Gherghiceanu M, Popescu BO. Triggers and effectors of oxidative stress at blood–brain barrier level: relevance for brain ageing and neurodegeneration. Oxid Med Cell Longev. 2013;2013:297512.
Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood–brain barrier. Hypertension. 2014;63:572–9.
Fleegal‐DeMotta MA, Doghu S, Banks WA. Angiotensin II modulates BBB permeability via activation of the AT1 receptor in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29:640–7.
Vital SA, Terao S, Nagai M, Granger DN. Mechanisms underlying the cerebral microvascular responses to angiotensin II‐induced hypertension. Microcirculation. 2010;17:641–9.
Yao ST, May CN. Intra‐carotid angiotensin II activates tyrosine hydroxylase‐expressing rostral ventrolateral medulla neurons following blood–brain barrier disruption in rats. Neuroscience. 2013;245:148–56.
Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood–brain barrier permeability via oxidative stress. Neuroscience. 2010;171:852–8.
Carnevale D, Mascio G, D’Andrea I, Fardella V, Bell RD, Branchi I, et al. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012;60:188–97.
Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med. 2003;139:761–76.
Rodriguez-Iturbe B, Pons H, Johnson RJ. Role of the immune system in hypertension. Physiol Rev. 2017;97:1127–64.
González-Marrero I, Castañeyra-Ruiz L, González-Toledo JM, Castañeyra-Ruiz A, de Paz-Carmona H, Castro R, et al. High blood pressure effects on the blood to cerebrospinal fluid barrier and cerebrospinal fluid protein composition: a two-dimensional electrophoresis study in spontaneously hypertensive rats. Int J Hypertens. 2013;2013:164653.
Shen XZ, Li Y, Li L, Shah KH, Bernstein KE, Lyden P, et al. Microglia participate in neurogenic regulation of hypertension. Hypertension. 2015;66:309–16.
Shi P, Raizada MK, Sumners C. Brain cytokines as neuromodulators in cardiovascular control. Clin Exp Pharm Physiol. 2010;37:e52–7.
Stern JE, Son S, Biancardi VC, Zheng H, Sharma N, Patel KP. Astrocytes contribute to angiotensin II stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension. 2016;68:1483–93.
Setiadi A, May CN, Yao ST. Ablation of astrocytes in the paraventricular nucleus disrupts the blood‐brain barrier and increases blood pressure in rats. FASEB J. 2017;31:1010.
Michalak Z, Lebrun A, Di Miceli M, Rousset MC, Crespel A, Coubes P, et al. IgG leakage may contribute to neuronal dysfunction in drug-refractory epilepsies with blood–brain barrier disruption. J Neuropathol Exp Neurol. 2012;71:826–38.
Meissner A, Minnerup J, Soria G, Planas AM. Structural and functional brain alterations in a murine model of Angiotensin II-induced hypertension. J Neurochem. 2017;140:509–21.
Yamazaki Y, Kanekiyo T. Blood–brain barrier dysfunction and the pathogenesis of Alzheimer’s disease. Int J Mol Sci. 2017;18:1965.
Sagare AP, Deane R, Zlokovic BV. Low-density lipoprotein receptor-related protein 1: a physiological Aβ homeostatic mechanism with multiple therapeutic opportunities. Pharm Ther. 2012;136:94–105.
Foulquier S, Namsolleck P, Van Hagen BT, Milanova I, Post MJ, Blankesteijn WM, et al. Hypertension-induced cognitive impairment: insights from prolonged angiotensin II infusion in mice. Hypertens Res. 2018;41:827.
Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, et al. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–42.
de la Torre JC, Stefano GB. Evidence that Alzheimer’s disease is a microvascular disorder: the role of constitutive nitric oxide. Brain Res Brain Res Rev. 2000;34:119–36.
Bueche CZ, Hawkes C, Garz C, Vielhaber S, Attems J, Knight RT, et al. Hypertension drives parenchymal β-amyloid accumulation in the brain parenchyma. Annals of Clinical and Translational. Neurology. 2014;1:124–9.
Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62:810–7.
Apostolova LG, Mosconi L, Thompson PM, Green AE, Hwang KS, Ramirez, et al. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol Aging. 2010;31:1077–88.
Bobinski M, Wegiel J, Wisniewski HM, Tarnawski M, Reisberg B, Mlodzik B, et al. Atrophy of hippocampal formation subdivisions correlates with stage and duration of Alzheimer disease. Dementia. 1995;6:205–10.
Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, et al. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64:1358–65.
Tomimoto H, Akiguchi I, Suenaga T, Nishimura M, Wakita H, Nakamura S, et al. Alterations of the blood–brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer’s disease patients. Stroke. 1996;27:2069–74.
Montagne A, Nation DA, Pa J, Sweeney MD, Toga AW, Zlokovic BV. Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol. 2016;131:687–707.
Kaya M, Kalayci R, Küçük M, Arican N, Elmas I, Kudat H, et al. Effect of losartan on the blood–brain barrier permeability in diabetic hypertensive rats. Life Sci. 2003;73:3235–44.
Ganten D, Hermann K, Bayer C, Unger T, Lang RE. Angiotensin synthesis in the brain and increased turnover in hypertensive rats. Science. 1983;221:869–71.
Guyenet P. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–46.
Reaux A, Fournie-Zaluski MC, David C, Zini S, Roques BP, Corvol P, et al. Aminopeptidase A inhibitors as potential central antihypertensive agents. Proc Natl Acad Sci USA. 1999;96:13415–20.
Gao J, Marc Y, Iturrioz X, Leroux V, Balavoine F, Llorens-Cortes C. A new strategy for treating hypertension by blocking the activity of the brain renin-angiotensin system with aminopeptidase A inhibitors. Clin Sci. 2014;127:135–48.
Tan J, Wang JM, Leenen FH. Inhibition of brain angiotensin converting enzyme by peripheral administration of trandolapril versus lisinopril in Wistar rats. Am J Hypertens. 2005;18:158–64.
Perret‐Guillaume C, Joly L, Jankowski P, Benetos A. Benefits of the RAS blockade: clinical evidence before the ONTARGET study. J Hypertens Suppl. 2009;27:S3–7.
Takeda S, Sato N, Takeuchi D, Kurinami H, Shinohara M, Niisato M, et al. Angiotensin receptor blocker prevented β-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension. 2009;54:6.
Pelisch N, Hosomi N, Ueno M, Nakano D, Hitomi H, Mogi M, et al. Blockade of AT1 receptors protects the blood–brain barrier and improves cognition in Dahl salt-sensitive hypertensive rats. Am J Hypertens. 2011;24:362–8.s.
Elkahloun AG, Hafko R, Saavedra JM. An integrative genome-wide transcriptome reveals that candesartan is neuroprotective and a candidate therapeutic for Alzheimer’s disease. Alzheimers Res Ther. 2016;28:5.
Davies NM, Kehoe PG, Ben-Shlomo Y, Martin RM. Associations of antihypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J Alzheimers Dis. 2011;26:699–708.
O’Caoimh R, Healy L, Gao Y, Svendrovski A, Kerins DM, Eustace J, et al. Effects of centrally acting angiotensin converting enzyme inhibitors on functional decline in patients with Alzheimer’s disease. J Alzheimers Dis. 2014;40:595–603.
Sink KM, Leng X, Williamson J, Kritchevsky SB, Yaffe K, Kuller L, et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med. 2009;169:1195–202.
Gao Y, O’Caoimh R, Healy L, Kerins DM, Eustace J, Guyatt G, et al. Effects of centrally acting ACE inhibitors on the rate of cognitive decline in dementia. BMJ Open. 2013;3:e002881.
Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, et al. PROGRESS Collaborative Group. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–75.
Takakura S, Satoh Y, Satoh H, Mori J, Kohsaka M. Effects of nilvadipine on regional cerebral blood flow and skin blood flow in anesthetized cats. Arch Int Pharmacodyn Ther. 1992;319:38–48.
Anderson C, Teo K, Gao P, Arima H, Dans A, Unger T, et al. ONTARGET and TRANSCEND Investigators. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011;10:43–53.
Hanyu H, Hirao K, Shimizu S, Sato T, Kiuchi A, Iwamoto T. Nilvadipine prevents cognitive decline of patients with mild cognitive impairment. Int J Geriatr Psychiatry. 2007;22:1264–6.
Amenta F, Lanari A, Mignini F, Silvestrelli G, Traini E, Tomassoni D. Nicardipine use in cerebrovascular disease: a review of controlled clinical studies. J Neurological Sci. 2009;283:219–23.
Paris D, Quadros A, Humphrey J, Patel N, Crescentini R, Crawford F, et al. Nilvadipine antagonizes both A beta vasoactivity in isolated arteries, and the reduced cerebral blood flow in APPSW transgenic mice. Brain Res. 2004;999:53–61.
Edvinsson L, Johansson BB, Larsson B, MacKenzie ET, Skärby T, Young AR. Calcium antagonists: effects on cerebral blood flow and blood–brain barrier permeability in the rat. Br J Pharm. 1983;79:141–8.
Nukhet Turkel A, Ziya Ziylan Y. Protection of blood–brain barrier breakdown by nifedipine in adrenaline-induced acute hypertension. Int J Neurosci. 2004;114:517–28.
Laurens C, Abot A, Delarue A, Knauf C. Central effects of beta-blockers may be due to nitric oxide and hydrogen peroxide release independently of their ability to cross the blood–brain barrier. Front Neurosci. 2019;13:33.
Gelber RP, Ross GW, Petrovitch H, Masaki KH, Launer LJ, White LR. Antihypertensive medication use and risk of cognitive impairment: the Honolulu-Asia Aging Study. Neurology. 2013;81:888–95.
Khachaturian AS, Zandi PP, Lyketsos CG, Hayden KM, Skoog I, Norton MC, et al. Antihypertensive medication use and incident Alzheimer disease: the Cache County Study. Arch Neurol. 2006;63:686–92.
Lenzsér G, Kis B, Bari F, Busija DW. Diazoxide preconditioning attenuates global cerebral ischemia-induced BBB permeability. Brain Res. 2005;1051:72–80.
Nishioku T, Takata F, Yamauchi A, Sumi N, Yamamoto I, Fujino A, et al. Protective action of indapamide, a thiazide-like diuretic, on ischemia-induced injury and barrier dysfunction in mouse brain microvascular endothelial cells. J Pharm Sci. 2007;103:323–7.
Buttler L, Jordão MT, Fragas MG, Ruggeri A, Ceroni A, Michelini LC. Maintenance of blood–brain barrier integrity in hypertension: a novel benefit of exercise training for autonomic control. Front Physiol. 2017;8:1048.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Katsi, V., Marketou, M., Maragkoudakis, S. et al. Blood–brain barrier dysfunction: the undervalued frontier of hypertension. J Hum Hypertens 34, 682–691 (2020). https://doi.org/10.1038/s41371-020-0352-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-020-0352-2
This article is cited by
-
Arterial hypertension in the chronic evolution of migraine: bystander or risk factor? An overview
The Journal of Headache and Pain (2024)
-
Methylglyoxal, a highly reactive dicarbonyl compound, as a threat for blood brain barrier integrity
Fluids and Barriers of the CNS (2023)
-
Predisposition to cortical neurodegenerative changes in brains of hypertension prone rats
Journal of Translational Medicine (2023)
-
Contributions of neuroimaging to the knowledge of the relationship between arterial hypertension and cognitive decline
Hypertension Research (2023)
-
Blood–Brain Barrier Dysfunction in Hypertensive Disorders of Pregnancy
Current Hypertension Reports (2023)