Abstract
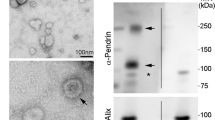
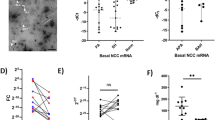
In primary aldosteronism (PA), the occurrence of K+ loss and hypertension suggest alterations in renal tubular transport, but the molecular basis of these alterations in humans is unclear. In this study, urinary extracellular vesicles (uEVs) isolated from patients undergoing fludrocortisone suppression testing (FST, as a means of confirming or excluding PA) were analyzed using mass spectrometry-based proteomics to determine the combined effects of an aldosterone analogue, NaCl and KCl supplementation on renal tubular protein abundance. Of quantified proteins, the Cl−/HCO3− exchanger pendrin decreased by a median 37% [−15, 57] (P < 0.01) and the potassium channel ROMK increased by a median 31% [−10, 85] (P < 0.01) during FST among 10 PA subjects. The trends remained, but to a lesser degree, in two subjects cured of PA by unilateral adrenalectomy. In PA subjects, plasma K+ increased from median 3.6 to 4.2 mM (P < 0.01) and 24 h urine K+ from 101 to 202 mmol (P < 0.01), while 24 h urine Na+/K+ decreased from 2.3 to 0.8 (P < 0.01). At baseline, pendrin negatively correlated with plasma K+ (P < 0.05) and positively correlated with plasma aldosterone (P < 0.01). There were no clear correlations between Δ pendrin (Δ = D4–D0) and changes in blood or urine variables, and no correlations between ROMK in any of the blood or urine variables either at baseline or during FST. We conclude that oral co-administration of mineralocorticoid and KCl in PA patients is associated with reduced pendrin and enhanced ROMK in uEVs. Pendrin reduction during FST suggests that the suppressive effects of oral KCl may outweigh pendrin upregulation by mineralocorticoids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The MS data have been deposited to the ProteomeXchange Consortium via the PRIDE (add reference: https://www.nature.com/articles/nbt.2839) partner repository with the dataset identifier PXD017083 (Reviewer account details: username: reviewer92263@ebi.ac.uk, password: 4PUMbpN2).
References
Briet M, Schiffrin EL. Vascular actions of aldosterone. J Vasc Res. 2013;50:89–99.
Pruthi D, McCurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol. 2014;34:355–64.
Galmiche G, Pizard A, Gueret A, El Moghrabi S, Ouvrard-Pascaud A, Berger S, et al. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension. 2014;63:520–6.
Yan Y, Wang C, Lu Y, Gong H, Wu Z, Ma X, et al. Mineralocorticoid receptor antagonism protects the aorta from vascular smooth muscle cell proliferation and collagen deposition in a rat model of adrenal aldosterone-producing adenoma. J Physiol Biochem. 2018;74:17–24.
Dinh QN, Young MJ, Evans MA, Drummond GR, Sobey CG, Chrissobolis S. Aldosterone-induced oxidative stress and inflammation in the brain are mediated by the endothelial cell mineralocorticoid receptor. Brain Res. 2016;1637:146–53.
Munoz-Durango N, Vecchiola A, Gonzalez-Gomez LM, Simon F, Riedel CA, Fardella CE, et al. Modulation of immunity and inflammation by the mineralocorticoid receptor and aldosterone. Biomed Res Int. 2015;2015:652738.
Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, et al. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Ren Physiol. 2001;280:F675–82.
Yang L, Frindt G, Lang F, Kuhl D, Vallon V, Palmer LG. SGK1-dependent ENaC processing and trafficking in mice with high dietary K intake and elevated aldosterone. Am J Physiol-Ren Physiol. 2017;312:F65–F76.
Conn JW. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med. 1955;45:3–17.
Conn JW, Cohen EL, Rovner DR, Nesbit RM. Normokalemic primary aldosteronism. A detectable cause of curable “essential” hypertension. JAMA. 1965;193:200–6.
Conn JW, Cohen EL, Rovner DR. Suppression of plasma renin activity in primary aldosteronism. JAMA. 1964;190:213–21.
Conn JW. The evolution of primary aldosteronism: 1954-1967. Harvey Lect. 1966;62:257–91.
Wu A, Wolley M, Stowasser M. The interplay of renal potassium and sodium handling in blood pressure regulation: critical role of the WNK-SPAK-NCC pathway. J Hum Hypertens. 2019;33:508–23.
Hoorn EJ, Gritter M, Cuevas CA, Fenton RA. Regulation of the renal NaCl cotransporter and its role in potassium homeostasis. Physiol Rev. 2020;100:321–56.
Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, et al. Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol. 2016;27:2436–45.
Huebner A, Somparn P, Benjachat T, Leelahavanichkul A, Avihingsanon Y, Fenton R, et al. Exosomes in urine biomarker discovery. In: Gao Y, editor. Urine proteomics in kidney disease biomarker discovery. Advances in experimental medicine and biology. 845: Netherlands: Springer; 2015. p. 43–58.
Spanu S, van Roeyen CR, Denecke B, Floege J, Muhlfeld AS. Urinary exosomes: a novel means to non-invasively assess changes in renal gene and protein expression. PLoS ONE. 2014;9:e109631.
Barros ER, Carvajal CA. Urinary exosomes and their cargo: potential biomarkers for mineralocorticoid arterial hypertension? Front Endocrinol. 2017;8:230.
Wolley MJ, Wu AH, Xu SX, Gordon RD, Fenton RA, Stowasser M. In primary aldosteronism, mineralocorticoids influence exosomal sodium-chloride cotransporter abundance. J Am Soc Nephrol. 2017;28:56–63.
Thompson A, Schafer J, Kuhn K, Kienle S, Schwarz J, Schmidt G, et al. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal Chem. 2003;75:1895–904.
Ross PL, Huang YLN, Marchese JN, Williamson B, Parker K, Hattan S, et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteom. 2004;3:1154–69.
Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J Clin Endocr Metab. 2016;101:1889–916.
Gheinani AH, Vogeli M, Baumgartner U, Vassella E, Draeger A, Burkhard FC, et al. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci Rep. 2018;8:3945.
Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–U60.
Lee JW, Chou CL, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol. 2015;26:2669–77.
Huebner AR, Cheng L, Somparn P, Knepper MA, Fenton RA, Pisitkun T. Deubiquitylation of protein cargo is not an essential step in exosome formation. Mol Cell Proteom. 2016;15:1556–71.
Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, et al. Pendrin modulates ENaC function by changing luminal HCO3. J Am Soc Nephrol. 2010;21:1928–41.
Soleimani M, Barone S, Xu J, Shull GE, Siddiqui F, Zahedi K, et al. Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc Natl Acad Sci USA. 2012;109:13368–73.
Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, et al. Reduced ENaC protein abundance contributes to the lower blood pressure observed in pendrin-null mice. Am J Physiol-Ren Physiol. 2007;293:F1314–F24.
Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet. 1997;17:411–22.
Taylor JP, Metcalfe RA, Watson PF, Weetman AP, Trembath RC. Mutations of the PDS gene, encoding pendrin, are associated with protein mislocalization and loss of iodide efflux: implications for thyroid dysfunction in Pendred syndrome. J Clin Endocrinol Metab. 2002;87:1778–84.
Pela I, Bigozzi M, Bianchi B. Profound hypokalemia and hypochloremic metabolic alkalosis during thiazide therapy in a child with Pendred syndrome. Clin Nephrol. 2008;69:450–3.
Lemoine S, Eladari D, Juillard L, Bonnefond A, Froguel P, Dubourg L. The Case | Hypokalemia and severe renal loss of sodium. Kidney Int. 2020;97:1305–6.
Grimm PR, Lazo-Fernandez Y, Delpire E, Wall SM, Dorsey SG, Weinman EJ, et al. Integrated compensatory network is activated in the absence of NCC phosphorylation. J Clin Investig. 2015;125:2136–50.
Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, et al. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension. 2003;42:356–62.
Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, et al. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution - Role in Cl- conservation. Hypertension. 2004;44:982–7.
Jacques T, Picard N, Miller RL, Riemondy KA, Houillier P, Sohet F, et al. Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension. J Am Soc Nephrol. 2013;24:1104–13.
Quentin F, Chambrey R, Trinh-Trang-Tan MM, Fysekidis M, Cambillau M, Paillard M, et al. The Cl-/HCO3- exchanger pendrin in the rat kidney is regulated in response to chronic alterations in chloride balance. Am J Physiol-Ren Physiol. 2004;287:F1179–F88.
Shibata S, Rinehart J, Zhang J, Moeckel G, Castaneda-Bueno M, Stiegler AL, et al. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab. 2013;18:660–71.
Kassirer JP, London AM, Goldman DM, Schwartz WB. On the pathogenesis of metabolic alkalosis in hyperaldosteronism. Am J Med. 1970;49:306–15.
Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–50.
Stowasser M, Gordon RD, Gunasekera TG, Cowley DC, Ward G, Archibald C, et al. High rate of detection of primary aldosteronism, including surgically treatable forms, after ‘non-selective’ screening of hypertensive patients. J Hypertens. 2003;21:2149–57.
Burrello J, Monticone S, Losano I, Cavaglia G, Buffolo F, Tetti M, et al. Prevalence of hypokalemia and primary aldosteronism in 5100 patients referred to a tertiary hypertension unit. Hypertension. 2020;75:1025–33.
Jones JW, Sebastian A, Hulter HN, Schambelan M, Sutton JM, Biglieri EG. Systemic and renal acid-base effects of chronic dietary potassium-depletion in humans. Kidney Int. 1982;21:402–10.
Cannon PJ, Ames RP, Laragh JH. Relation between potassium balance and aldosterone secretion in normal subjects and in patients with hypertensive or renal tubular disease. J Clin Investig. 1966;45:865.
Nakamura S, Amlal H, Galla JH, Soleimani M. NH4+ secretion in inner medullary collecting duct in potassium deprivation: role of colonic H+-K+-ATPase. Kidney Int. 1999;56:2160–7.
Hernandez RE, Schambelan M, Cogan MG, Colman J, Morris RC Jr., Sebastian A. Dietary NaCl determines severity of potassium depletion-induced metabolic alkalosis. Kidney Int. 1987;31:1356–67.
Dubose TD, Good DW. Effects of chronic hyperkalemia on renal production and proximal tubule transport of ammonium in rats. Am J Physiol. 1991;260:F680–F7.
Dubose TD, Good DW. Chronic hyperkalemia impairs ammonium transport and accumulation in the inner medulla of the rat. J Clin Investig. 1992;90:1443–9.
Xu N, Hirohama D, Ishizawa K, Chang WX, Shimosawa T, Fujita T, et al. Hypokalemia and pendrin induction by aldosterone. Hypertension. 2017;69:855.
van der Lubbe N, Jansen PM, Salih M, Fenton RA, van den Meiracker AH, Danser AJ, et al. The phosphorylated sodium chloride cotransporter in urinary exosomes is superior to prostasin as a marker for aldosteronism. Hypertension. 2012;60:741–8.
Acknowledgements
AW is supported by a scholarship from the Commonwealth Government of Australia. This work is supported by a grant from the Leducq Foundation (Potassium in Hypertension Network). Further funding to RAF is provided by the Novo Nordisk Foundation and the Danish Independent Research Fund: Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MS is currently Editor-in-Chief of the Journal of Human Hypertension, necessitating handling of the manuscript by one of the other co-editors. The remaining authors declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, A., Wolley, M.J., Wu, Q. et al. The Cl−/HCO3− exchanger pendrin is downregulated during oral co-administration of exogenous mineralocorticoid and KCl in patients with primary aldosteronism. J Hum Hypertens 35, 837–848 (2021). https://doi.org/10.1038/s41371-020-00439-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-020-00439-7
This article is cited by
-
Urinary extracellular vesicles in childhood kidney diseases
Pediatric Nephrology (2023)
-
Urinary extracellular vesicle mRNA analysis of sodium chloride cotransporter in hypertensive patients under different conditions
Journal of Human Hypertension (2022)
-
Characterization of pendrin in urinary extracellular vesicles in a rat model of aldosterone excess and in human primary aldosteronism
Hypertension Research (2021)