Abstract
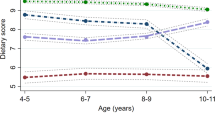
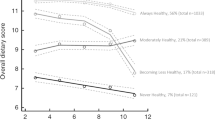
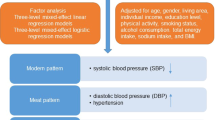
A healthy diet reduces risk for high blood pressure. A small body size at birth increases risk for high blood pressure. Our aim was to study whether birth weight modifies the association between a healthy Nordic diet, characterized by high intake of Nordic vegetables, fruits, and berries, whole-grain rye, oat, and barley, and rapeseed oil, and blood pressure. Finnish men and women (n = 960) born in 1934–1944 attended clinical visits including clinical measurements, and questionnaires in 2001–2004 and 2011–2013. Linear regression was applied to investigate the interactions between birth weight and Nordic diet (measured by the Baltic sea diet score (BSDS)) on blood pressure change during the 10-year follow-up. Baseline Nordic diet and birth weight showed a significant interaction on systolic blood pressure (SBP) (p = 0.02), and pulse pressure (PP) (p < 0.01) over a 10-year follow-up. In the lowest birth weight category (women < 2951 g, men < 3061 g), predicted SBP decreased across BSDS thirds (lowest (T1): 155 mmHg, highest (T3): 145 mmHg, p for linearity = 0.01) as did predicted PP (T1: 71 mmHg, T3: 63 mmHg, p < 0.01). In the middle birth weight category, predicted SBP increased across BSDS thirds (T1: 151 mmHg, T3: 155 mmHg, p = 0.02) as did predicted PP (T1: 67 mmHg, T3: 71 mmHg, p < 0.01). In the highest birth weight category, no associations were found. Higher adherence to a healthy Nordic diet was associated with lower SBP and PP in individuals with low birth weight but with higher SBP and PP in those with average birth weight.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The dataset and code supporting the conclusions of this article is available upon a reasonable request from the authors.
References
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60.
Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:38.
Gamborg M, Byberg L, Rasmussen F, Andersen PK, Baker JL, Bengtsson C, et al. Birth weight and systolic blood pressure in adolescence and adulthood: meta-regression analysis of sex- and age-specific results from 20 Nordic studies. Am J Epidemiol. 2007;166:634–45.
Zhang Z, Kris-Etherton PM, Hartman TJ. Birth weight and risk factors for cardiovascular disease and type 2 diabetes in US children and adolescents: 10 year results from NHANES. Matern Child Health J. 2014;18:1423–32.
Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int. 2001;59:1663–9.
Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: the role of fetal programming. Hypertension. 2006;47:502–8.
Gurusinghe S, Tambay A, Sethna CB. Developmental origins and nephron endowment in hypertension. Front Pediatr. 2017;5:151.
Franco MC, Christofalo DM, Sawaya AL, Ajzen SA, Sesso R. Effects of low birth weight in 8- to 13-year-old children: implications in endothelial function and uric acid levels. Hypertension. 2006;48:45–50.
Levitt NS, Lambert EV, Woods D, Seckl JR, Hales CN. Adult BMI and fat distribution but not height amplify the effect of low birthweight on insulin resistance and increased blood pressure in 20-year-old South Africans. Diabetologia. 2005;48:1118–25.
Eriksson JG, Forsen TJ, Kajantie E, Osmond C, Barker DJ. Childhood growth and hypertension in later life. Hypertension. 2007;49:1415–21.
Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith GD. Birthweight, body-mass index in middle age, and incident coronary heart disease. Lancet. 1996;348:1478–80.
Rajaleid K, Janszky I, Hallqvist J. Small birth size, adult overweight, and risk of acute myocardial infraction. Epidemiology. 2011;22:138–47.
Li Y, Ley SH, VanderWeele TJ, Curhan GC, Rich-Edwards JW, Willett WC, et al. Joint association between birth weight at term and later life adherence to a healthy lifestyle with risk of hypertension: a prospective cohort study. BMC Med. 2015;13:175–1.
Perala MM, Moltchanova E, Kaartinen NE, Mannisto S, Kajantie E, Osmond C, et al. The association between salt intake and adult systolic blood pressure is modified by birth weight. Am J Clin Nutr. 2011;93:422–6.
Ramezani-Jolfaie N, Mohammadi M, Salehi-Abargouei A. The effect of healthy Nordic diet on cardio-metabolic markers: a systematic review and meta-analysis of randomized controlled clinical trials. Eur J Nutr. 2018;58:2159–74.
Kanerva N, Kaartinen NE, Rissanen H, Knekt P, Eriksson JG, Saaksjarvi K, et al. Associations of the Baltic sea diet with cardiometabolic risk factors-a meta-analysis of three Finnish studies. Br J Nutr. 2014;112:616–26.
Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–24.
Adamsson V, Reumark A, Cederholm T, Vessby B, Riserus U, Johansson G. What is a healthy Nordic diet? Foods and nutrients in the NORDIET study. Food Nutr Res. 2012;56. https://doi.org/10.3402/fnr.v56i0.18189.
Mannisto S, Virtanen M, Mikkonen T, Pietinen P. Reproducibility and validity of a food frequency questionnaire in a case-control study on breast cancer. J Clin Epidemiol. 1996;49:401–9.
Paalanen L, Mannisto S, Virtanen MJ, Knekt P, Rasanen L, Montonen J, et al. Validity of a food frequency questionnaire varied by age and body mass index. J Clin Epidemiol. 2006;59:994–1001.
Reinivuo H, Hirvonen T, Ovaskainen ML, Korhonen T, Valsta LM. Dietary survey methodology of FINDIET 2007 with a risk assessment perspective. Public Health Nutr. 2010;13:915–9.
Kanerva N, Kaartinen NE, Schwab U, Lahti-Koski M, Mannisto S. The Baltic sea diet score: a tool for assessing healthy eating in Nordic countries. Public Health Nutr. 2014;17:1697–705.
Playdon MC, Moore SC, Derkach A, Reedy J, Subar AF, Sampson JN, et al. Identifying biomarkers of dietary patterns by using metabolomics. Am J Clin Nutr. 2017;105:450–65.
Simonetti GD, Raio L, Surbek D, Nelle M, Frey FJ, Mohaupt MG. Salt sensitivity of children with low birth weight. Hypertension. 2008;52:625–30.
Ruys CA, Rotteveel J, van de Lagemaat M, Lafeber HN, Finken MJJ. Salt sensitivity of blood pressure at age 8 years in children born preterm. J Hum Hypertens. 2018;32:367–76.
Leon DA, Koupilova I, Lithell HO, Berglund L, Mohsen R, Vagero D, et al. Failure to realise growth potential in utero and adult obesity in relation to blood pressure in 50 year old Swedish men. BMJ. 1996;312:401–6.
Hardy R, Wadsworth ME, Langenberg C, Kuh D. Birthweight, childhood growth, and blood pressure at 43 years in a British birth cohort. Int J Epidemiol. 2004;33:121–9.
Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13.
Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000;160:1085–9.
Souza LV, De Meneck F, Oliveira V, Higa EM, Akamine EH, Franco MDC. Detrimental impact of low birth weight on circulating number and functional capacity of endothelial progenitor cells in healthy children: role of angiogenic factors. J Pediatr. 2019;206:72–77.e1.
Stsiapura VI, Bederman I, Stepuro II, Morozkina TS, Lewis SJ, Smith L, et al. S-Nitrosoglutathione formation at gastric pH is augmented by ascorbic acid and by the antioxidant vitamin complex, Resiston. Pharm Biol. 2018;56:86–93.
Stanhewicz AE, Kenney WL. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr Rev. 2017;75:61–70.
Oak MH, Auger C, Belcastro E, Park SH, Lee HH, Schini-Kerth VB. Potential mechanisms underlying cardiovascular protection by polyphenols: role of the endothelium. Free Radic Biol Med. 2018;122:161–70.
Sibmooh N, Piknova B, Rizzatti F, Schechter AN. Oxidation of iron-nitrosyl-hemoglobin by dehydroascorbic acid releases nitric oxide to form nitrite in human erythrocytes. Biochemistry. 2008;47:2989–96.
Romeu M, Aranda N, Giralt M, Ribot B, Nogues MR, Arija V. Diet, iron biomarkers and oxidative stress in a representative sample of Mediterranean population. Nutr J. 2013;12:102.
Funding
This study was supported by grants from Finska Läkaresällskapet, the Finnish Special Governmental Subsidy for Health Sciences, Academy of Finland (127437, 129306, 130326, 134791, 263924, and 315690), Samfundet Folkhälsan, Liv och Hälsa, EU FP7 [Developmental Origins of Healthy Aging (DORIAN)] project number 278603, and EU H2020-PHC-2014-DynaHealth grant 633595, Horizon2020 award No. 733206 LifeCycle. (all for the Helsinki Birth Cohort Study), European Commission, Horizon2020 award 733280 RECAP), Foundation for Cardiovascular Research, Foundation for Diabetes Research, Foundation for Pediatric Research, Novo Nordisk Foundation, Signe and Ane Gyllenberg Foundation, Sigrid Jusélius Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41371_2020_423_MOESM1_ESM.docx
Supplemental Table S1. Coefficient, 95% CI, and p-values for missingness of use of blood pressure affecting medication data at follow-up (2011-2013) (0=data not missing/1=data missing) by one unit increase in a given variable (n=955).
41371_2020_423_MOESM2_ESM.docx
Supplemental Table S2. Coefficient, 95% CI, and p-values for missingness of maternal pre-delivery BMI data (1=missing data/0=data not missing) by one unit increase of a given variable (n=955).
41371_2020_423_MOESM3_ESM.docx
Supplemental Figure S3. Proportions of observed (n=823), imputed (n=137), and completed (n=960) values of blood pressure affecting medication use at follow-up (0 = data not missing, 1 = data missing) in each 10 imputed datasets. Multiple imputation (10 imputations) with logistic regression model included covariates age, sex, educational attainment, gestational age, smoking, leisure-time physical activity, BMI, weight change during follow-up, energy intake, Baltic sea diet score, sodium intake, use of blood-pressure affecting medication at baseline, household income, systolic blood pressure at baseline and at follow-up, diastolic blood pressure at baseline and at follow-up, and maternal pre-pregnancy BMI.
41371_2020_423_MOESM4_ESM.docx
Supplemental Figure S4. Proportions of observed (n=842), imputed (n=137), and completed (n=960) values of maternal pre-delivery BMI (0 = data not missing, 1 = data missing) in each 10 imputed datasets. Multiple imputations (10 imputations) with regression model included covariates age, sex, educational attainment, gestational age, smoking, leisure-time physical activity, BMI, weight change during follow-up, energy intake, Baltic sea diet score, sodium intake, use of blood-pressure affecting medication at baseline, household income, systolic blood pressure at baseline and at follow-up, diastolic blood pressure at baseline and at follow-up, and blood pressure affecting medication use during follow-up.
41371_2020_423_MOESM5_ESM.docx
Supplemental Table S5. F-values (df1, df2) and p-values for interaction between birth weight and Baltic sea diet score on blood pressure parameters at baseline (2001-2003).
41371_2020_423_MOESM6_ESM.docx
Supplemental Table S6. Association between baseline BSDS and blood pressure parameters at follow-up by BW in observed cases and completed cases (multiple imputation). F-values (f1, df2) and p-values for interaction term (BW x BSDS) on blood pressure parameters and predicted blood pressure parameters in thirds of Baltic sea diet score by birth weight.
41371_2020_423_MOESM7_ESM.docx
Supplemental Table S7. Regression coefficients and their 95 % confidence intervals for the association between BSDS components at baseline and systolic blood pressure and pulse pressure in 10-year follow-up.
41371_2020_423_MOESM8_ESM.docx
Supplemental Figure S8. Linear predictions of systolic blood pressure across birth weight deciles in thirds of the Baltic sea diet score. BSDS, Baltic sea diet score.
Rights and permissions
About this article
Cite this article
Meinilä, J., Perälä, MM., Kanerva, N. et al. Birth weight modifies the association between a healthy Nordic diet and office blood pressure in old age. J Hum Hypertens 35, 849–858 (2021). https://doi.org/10.1038/s41371-020-00423-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-020-00423-1