Abstract
Background
Dried blood spot (DBS) sampling is a simple, cost-effective, and minimally invasive alternative to venipuncture for measuring exposure biomarkers in public health and epidemiological research. DBS sampling provides advantages in field-based studies conducted in low-resource settings and in studies involving infants and children. In addition, DBS samples are routinely collected from newborns after birth (i.e., newborn dried blood spots, NDBS), with many states in the United States permitting access to archived NDBS samples for research purposes.
Objectives
We review the state of the science for analyzing exposure biomarkers in DBS samples, both archived and newly collected, and provide guidance on sample collection, storage, and blood volume requirements associated with individual DBS assays. We discuss recent progress regarding analytical methods, analytical sensitivity, and specificity, sample volume requirements, contamination considerations, estimating extracted blood volumes, assessing stability and analyte recovery, and hematocrit effects.
Methods
A systematic search of PubMed (MEDLINE), Embase (Elsevier), and CINAHL (EBSCO) was conducted in March 2022. DBS method development and application studies were divided into three main chemical classes: environmental tobacco smoke, trace elements (including lead, mercury, cadmium, and arsenic), and industrial chemicals (including endocrine-disrupting chemicals and persistent organic pollutants). DBS method development and validation studies were scored on key quality-control and performance parameters by two members of the review team.
Results
Our search identified 47 published reports related to measuring environmental exposure biomarkers in human DBS samples. A total of 28 reports (37 total studies) were on methods development and validation and 19 reports were primarily the application of previously developed DBS assays. High-performing DBS methods have been developed, validated, and applied for detecting environmental exposures to tobacco smoke, trace elements, and several important endocrine-disrupting chemicals and persistent organic pollutants. Additional work is needed for measuring cadmium, arsenic, inorganic mercury, and bisphenol A in DBS and NDBS samples.
Significance
We present an inventory and critical review of available assays for measuring environmental exposure biomarkers in DBS and NDBS samples to help facilitate this sampling medium as an emerging tool for public health (e.g., screening programs, temporal biomonitoring) and environmental epidemiology (e.g., field-based studies).
Similar content being viewed by others
Introduction
Human biomonitoring has found a prominent role in investigating relationships between environmental exposures and adverse health outcomes. Major government tracking studies, such as the National Health and Nutrition Examination Study (NHANES), have measured key components of the human exposome in blood and urine using biomarker measurements to retrospectively assess exposures, and prospectively interpret disease states on a population level [1, 2]. Myriads of smaller studies have focused on specific links between environmental exposures and disease using combinations of blood, breath, lavage fluids, adipose tissues, and urine as the biological media for informing the exposure to risk paradigm [3,4,5,6]. Unlike environmental measurements (e.g., measuring pollutants in air and drinking water), biomarker measurements can be relatively invasive. While medical patients may be willing to provide repeated blood draws and collection of their urine, the general public is not so acquiescent in allowing biological monitoring for indirect purposes of public health assessment. As such, the value of environmental biomonitoring is best supported with the least invasive, simplest sampling methods in the field, with perhaps more complex analyses reserved for the laboratory [7].
Blood analysis has often been considered the “gold standard” for human exposure and disease diagnostics [8]. However, the collection of venous blood is relatively invasive and requires trained medical personnel, costly refrigeration and shipping, and special laboratory processing and handling [9]. Dried blood spot (DBS) samples are 4–5 drops of whole blood from a minimally invasive finger- or heel-prick, absorbed onto specially designed filter paper (e.g., Whatman 903). DBS samples can be shipped at ambient temperatures in flat envelopes [9], since the United States Postal Service considers DBS samples a Nonregulated Infectious Material. DBS samples are also routinely collected from newborns after birth (i.e., newborn dried blood spots, NDBS) to screen for inborn errors of metabolism and other treatable disorders, and many states in the United States permit access to residual NDBS samples for research purposes. As a result, DBS sampling represents a large and invaluable resource for assessing exposures to environmental toxicants. In addition, DBS sampling allows for self-collection [10, 11], which is an important advantage of this approach during the COVID-19 pandemic. Because of these advantages, DBS sampling is particularly well suited for population-based studies involving younger children and infants, such as the Environmental Influences on Child Health Outcomes (ECHO) program [12, 13]. While these advantages have motivated the use of DBS sampling in several recent large-scale health surveys in the US and globally [14,15,16], the use of DBS sampling for estimating exposures to chemical toxicants in epidemiological research has recently accelerated within the scientific community, with the publication of many new validated environmental biomarker assays [17, 18].
The utility of DBS for newborn screening was first demonstrated by Robert Guthrie for the testing of phenylketonuria in infants in the early 1960s [19]. Since this time, the use NDBS for screening infants for metabolic disorders has greatly expanded, and routine screening is now standard practice for all US hospitals. This process was accelerated by the introduction of tandem mass spectrometry (MS) in the 1990s, which fostered a new era where large panels of biomarkers could be simultaneously measured in a single analysis [20]. In the US, 35 primary health conditions and 26 recommended secondary targets are included in the Nationally Recommended Uniform Screening Panel by the American College of Medical Genetics [21]. Storage policies and conditions for retaining residual NDBS samples, however, differ widely between states. These differences are often centered around the ethical issues of using archived NDBS without informed parental consent. As a result, many states have chosen to not retain and store residual NDBS samples in the interest of preserving patients’ privacy, while other states retain NDBS specimens for extended timeframes which can be used for research purposes [22]. In addition, even when residual NDBS are retained by states, the cost of storing samples is a significant barrier and can result in suboptimal storage conditions (e.g., storage at room temperature and/or without the use of desiccant). Consequently, NDBS samples are more susceptible to factors such as background contamination and sample degradation. In contrast, DBS samples collected in the field are more carefully handled under standardized research conditions to minimize factors that might influence sample quality.
While DBS provide many advantages over venipuncture, measuring biomarkers in DBS samples poses several challenges, including small and variable blood volumes, requirements for continued lab- and field-based quality assurance measures, validation with gold standard, and higher sample complexity compared to plasma/serum samples. In addition, the stability of biomarkers in DBS samples can be an issue and volatile compounds can be lost during the drying process. Many immunoassay-based methods have been developed; however, these assays tend to have high reagent costs and require long development times. Immunoassays may provide the advantages of high sample throughput and analytical sensitivity but can lack biomarker specificity giving rise to measurement error [23]. MS-based assays provide some advantages because they account for some of these challenges. For example, solid phase extraction and chromatographic separation can be coupled with MS to reduce sample complexity [24]. While MS does not necessarily resolve the issue of assay cost, MS-based assays can be easily multiplexed and provide high biomarker specificity.
When discussing recent progress in the DBS field, limitations and challenges associated with quantifying exposure biomarkers in DBS samples must be considered on a biomarker-by-biomarker basis. This is because sensitivity, specificity, stability, and contamination issues can differ greatly between individual biomarkers [25]. Here, we present a state-of-the-science review for measuring biomarkers in DBS to estimate exposures to environmental toxicants. This review is meant to act as a guide for researchers interested in using DBS in environmental health studies, with a focus on protocols that have been extensively developed and well validated. Details on required sample volumes, biomarker stability, and other important details related to sample collection, shipment, and storage are discussed. By identifying key DBS methods categorized by chemical classes of environmental toxicants, we present an inventory of available assays that will guide the use of DBS sampling in population- and community-based research.
Methods
In this review, a search of three bibliographic databases was conducted. The search was designed to identify all articles on DBS sampling, biomarkers, and environmental exposures (Fig. 1). A research librarian (DAN) collaboratively developed the search strategies with the review author (TAJ), and on March 7, 2022, searched PubMed (MEDLINE), Embase (Elsevier), and CINAHL (EBSCO). A full list of search strategies and terms is provided in the Supplementary information (SI). Two reviewers (TAJ and JSK) screened the results in duplicate according to pre-determined inclusion criteria using the screening platform, Rayyan. To meet our inclusion criteria (more details: SI), studies had to use DBS sampling to measure biomarkers of internal doses of exposures to exogenous pollutants (i.e., native exogenous compounds and/or their metabolites) in human blood samples. Biomarkers of response were excluded (Fig. 2). DBS method development and validation studies were evaluated based on key quality-control parameters and performance metrics outlined by McDade (2014) [9]. Method development and validation studies were scored by a member of the review team (TAJ, YB, RI, and NDM) and spot checked (TAJ, YB, RI, NDM, and JSK) after being extracted and inputted into Table 1.
Results
Using the search terms provided in the SI, a total of 2615 reports were found across all databases and relevant reviews. After deduplication, 1620 reports were screened on the basis of titles and abstracts (Fig. 1). The full texts of 61 reports were screened and 23 reports were excluded. Of the reports that did not measure an environmental exposure biomarker, most used DBS sampling to measure non-specific markers of internal biological response (i.e., inflammation, oxidative stress, or cholinesterase depression) to environmental exposures. After full-text review, 38 reports met the inclusion criteria. In addition, 9 reports were identified by searching reference lists of included studies (47 total reports included).
We highlight key quality-control and performance parameters for each exposure biomarker in Table 1, and we summarize key details from application studies in Table 2. We highlight the estimated blood volumes for different DBS punch sizes in Fig. 3. Of the 47 reports that met our inclusion criteria, 28 reports were categorized as primarily method development and validation (n = 7 environmental tobacco smoke (ETS), 12 trace elements, 5 endocrine-disrupting chemicals (EDCs)/persistent organic pollutants (POPs), and 4 other environmental exposure biomarkers) and 19 reports were categorized as being primarily application of previously developed DBS assays (e.g., population-based studies or temporal biomonitoring). However, many method development reports include applications of assays in relatively small sample sizes, while many application-based reports include method and field validation for continued quality assurance.
The image shows the size of each spot as a function of the volume of blood applied to the filter paper (50–70 µL). The range between 50 and 70 µL corresponds with the typical volume of a single drop of blood collected by finger- or heel-prick. The punches shown on the first three spots show the number of discs that can be removed based on commonly used disc sizes (i.e., 3.2-mm, ~3.2 µL whole blood; 4.7-mm, ~6.9 µL whole blood; and 6.0-mm, ~11.2 µL whole blood; see Supplementary information for more disc-blood volume estimates).
Environmental tobacco smoke
Exposure to ETS poses significant health risks for infants and children, including decreased lung growth, and increased risk of respiratory infections, otitis media, and childhood asthma [26]. Second-hand tobacco smoke (SHS) exposure among non-smokers can also result in adverse health outcomes, including cardiovascular disease and lung cancer, with higher exposure-response relationships at lower levels of exposure [27]. Previous methods for estimating prenatal and postnatal exposures to ETS have relied on administering questionnaires to parents [26], which is subject to both recall and social desirability biases.
Cotinine, a primary metabolite of nicotine, is a sensitive and specific biomarker of exposure to first- and second-hand tobacco smoke and can be quantified in DBS samples. While nicotine has a biological half-life of less than 3 h, cotinine has a biological half-life of 15–20 h [28]. Thus, cotinine persists in the blood stream for longer than nicotine and is the gold standard biomarker for exposure to ETS in blood. Cotinine is further metabolized by P450 2A6 to trans-3’-hydroxycotinine (3’-HCOT) at rates that vary across people. Consequently, some investigators have used the ratio of 3’-HCOT to cotinine to account for variability in nicotine metabolism [28, 29].
Overview
A total of nine studies (seven published reports) were conducted on methods development and validation. Of these, six reports were from the US and one was from Germany. Three studies used NDBS samples [30,31,32] and six studies used DBS samples [28, 29, 33, 34]. Eight studies used human participant samples and one used reference materials with human blood from volunteers [29]. Two studies measured cotinine in matched plasma samples [28, 34] and one study used NDBS and matched umbilical cord blood samples [31]. Four studies reported detection frequencies [28, 31, 34] and two reported sensitivity and specificity in accurately predicting maternal smoking status [31, 34]. These assays were applied in two larger-scale studies involving 1541 DBS samples collected from children during routine lead screening [35] and 1414 archived NDBS samples collected from several states [36].
Methods development
Early methods for quantifying cotinine in DBS were not sensitive enough to detect or quantify fetal exposure to SHS [30, 31, 33]. In 2013, a revised method using liquid chromatography-mass spectrometry (LC-MS) was described, with greater analytical sensitivity and precision (limit of quantification (LOQ) of 0.3 ng/g)) and excellent correlation with plasma cotinine levels [28]. This revised method normalized measurements according to excised DBS mass to reduce variability due to hematocrit effects and included quantification of 3’-HCOT to account for variations in nicotine metabolism [28]. This study also analyzed the effects of storage time and conditions on cotinine measurements by comparing subsets of samples stored at either −20 °C or room temperature (20 °C), 11–26 months apart. The study reported no effects of storage time or condition on cotinine measurements [28]. Another assay using LC-MS was validated (LOQ of 3.13 ng/mL) with a strong correlation between cotinine levels in archived NDBS samples and in umbilical cord blood with high sensitivity and specificity in predicting maternal smoking status shortly before birth [31]. This study also reported negligible effects of storage time and conditions on cotinine measurements by analyzing subsamples stored in dark and room temperature for 7 months [31]. Although NDBS values were highly correlated with umbilical cord blood cotinine levels, they were on average 15.5 ng/mL lower [31]. This bias was more pronounced when DBS samples were collected >2 days after birth [31]. An automated extraction procedure to enable high throughput analyses of nicotine, cotinine, and 3’-HCOT has also been described [29]. This study reported negligible hematocrit effects for levels ranging from 30 to 60% [29].
Recently, an ultra-sensitive (LOQ < 0.25 ng/mL), high throughput method for quantifying cotinine in plasma and reconstituted DBS samples of smokers and non-smokers was developed [34]. This method utilized a single 3.2-mm DBS punch (estimated ~5 µL blood) and used DBS-based calibration standards to account for matrix effects [34]. DBS cotinine levels were highly correlated with matched plasma samples and had high sensitivity and specificity in distinguishing smokers from non-smokers [34]. Hematocrit effects were negligible [34]. This assay was applied to 50 archived DBS samples (with unknown smoking status) collected via finger-prick from infants and children ages 0–21 years old [34]. In total, 7 out of the 50 samples had levels of cotinine above the assay’s LOQ [34].
Guidance
The assays developed by Ladror et al. [34] and Murphy et al. [28] have the highest sensitivities (LOQ ~0.25 ng/mL) and require the least amount of sample volumes (e.g., 3.2-mm punches for high-exposure groups or 4.8-mm punches for low-exposure groups). These assays have been validated on key quality-control metrics (Table 1) and have been developed for high sample throughput. This level of analytical sensitivity is sufficient to quantify the 90th percentile of serum cotinine among non-smokers in the US (0.305–0.356 ng/mL) [37]. Hematocrit effects have been investigated by three studies and have been found to be negligible [29, 33, 34]. Cotinine concentrations were reported to be stable in DBS and NDBS samples for at least 7–10 months at room temperature [28, 31] and up to 4 years at 4 °C (small sample size) [33]. Additional research is needed into potential matrix effects, including whole blood (DBS) versus plasma/serum [34]. Duplicate testing on positive DBS values <10 ng/mL is recommended to minimize false positive results [31]. The optimal point on the receiver operating curve to differentiate active smoking versus non-smoking status in DBS samples is 6 ng/mL [31]. Based on the high analytical sensitivities, sample throughput, small sample volume requirements, and high-quality-control parameters of developed assays for measuring cotinine in DBS samples, these assays appear ready for use in large-scale population-based studies and public health screening programs.
Applications
DBS approaches have been applied to two large pediatric cohort studies to detect cotinine in extant DBS samples collected during routine lead screening [35, 36]. Both of these studies used a previously described and validated assay with a limit of detection (LOD) of 0.3 ng/g (~0.2 ng/mL blood) [28]. Significantly higher cotinine levels were independently associated with African American race, older age, Medicaid coverage, higher state smoking rates, and higher average winter temperatures [35]. Cotinine levels were detected in 61% of DBS samples and were strongly associated with elevated blood lead levels in DBS samples [35].
The assay developed by Murphy et al. was also applied to an observational, cross-sectional study with a large collection of newborn DBS samples from screening programs in California, Michigan, New York, and Washington [36]. Cotinine levels (>0.3 ng/g) were detected in 35% of newborn DBS samples, and higher levels were associated with African American race due to environmental racism, racist advertising policies, and residential segregation [36, 38]. This study also found evidence of non-disclosure among mothers: cotinine levels suggesting active smoking status of the mother (>9.0 ng/g) were found in 12% of NDBS samples, despite 41% of these mothers reporting that they did not smoke during pregnancy [36]. These findings support bias in self-report smoking data, which would underestimate the true impact of ETS exposure on health outcomes.
Trace elements
Prenatal and childhood exposure to trace elements, including arsenic (As), lead (Pb), mercury (Hg), and cadmium (Cd), are a significant public health concern. Here, we focus on As, Pb, Hg, and Cd because they are listed as the first, second, third, and seventh most hazardous substances on the Agency for Toxic Substances and Disease Registry’s 2019 CERCLA priority list of 275 substances, respectively. Exposure to Pb, Cd, and As has also been implicated in the progression of cardiovascular disease [39] and chronic exposure to low levels of Pb has been linked to cognitive and behavioral disturbances in children [40]. Up until 2012, children were identified as having a blood lead “level of concern” with values >10 µg/dL [41]. The CDC has revised its guidelines to consider any value >3.5 µg/dL a blood lead “reference value” that puts the child in the 97.5th percentile of blood lead levels among US children 1–5 years old [41]. This value is not health-based, and there is no established safe level of lead exposure in children.
Exposures to Hg, As, and Cd are also a major health concern and deserve special attention. Human exposure to methyl-Hg occurs primarily through the dietary consumption of marine fish and other seafood. Methyl-Hg readily crosses the placenta and passes through the fetal blood–brain barrier. Chronic, low-level exposure to methyl-Hg, especially in utero and in the first 2 years of life, may increase the risk for neurologic and psychiatric conditions later in life [42, 43]. Human exposure to As may occur through drinking from contaminated water sources and from dietary consumption. Arsenic has been associated with an increased risk for cancers of the skin, lung, bladder, kidney, and liver—with early-life susceptibility [44]. Similarly, Cd exposure occurs primarily through consumption of contaminated food and water, as well as from the inhalation of cigarette smoke [45]. Observational studies have linked Cd exposure with an increased risk for cancers of the breast, lung, prostate, nasopharynx, pancreas, and kidney—with the kidney and liver being especially susceptible organs [45].
Overview
A total of 16 studies (12 published reports) were primarily related to method development and validation for measuring exposure to Pb, Hg, Cd, and As in DBS samples. Of these, eight published reports were from the US, two were from Canada [46, 47], one was from Canada/Tanzania [48], and one was from Germany [49]. Seven studies used NDBS samples. One study compared NDBS measurements to paired whole blood levels [50], one study compared NDBS measurements with cord blood [51], and six studies compared DBS measurements with gold standard venous blood values [46,47,48, 52].
Methods development
The historical development of DBS assays that quantify Pb and other trace elements has been succinctly summarized by a recent review [18]. Here, we will highlight the main developments that apply to Pb, Hg, Cd, and As before discussing each individually. Because standard filter paper used for collecting DBS samples is not designed for trace elemental analyses, contamination is a concern. Trace element contamination can be inherent in the filter paper matrix, and can also occur before, during, and after the blood is collected on the filter paper [52]. In addition, trace element contamination is not homogenously distributed across the card, and therefore performing blank filter paper subtractions using sections of the filter paper adjacent to the blood spot does not work well with low levels of environmental exposure [52, 53]. To address this issue, Funk et al. pretreated the filter paper using a combination of acids to remove contamination prior to DBS sample collection, which vastly improved the agreement between DBS measurements and matched “gold standard” venous blood samples for Pb, Hg, Cd, and As [52]. While this approach cannot be applied when using existing stored samples (e.g., NDBS), it can be used in prospective studies [52]. Funk et al. also evaluated analyte stability and recovery across collection years and found no significant effects of storage time on recovery rates for Pb, Hg, Cd, and As among archived NDBS samples [53].
Lead
Recently developed assays for measuring Pb in DBS samples have improved upon prior methods [54,55,56,57,58,59,60,61]. Nyanza et al. developed and validated the most sensitive methods for measuring Pb in field-collected DBS samples using ICP-MS [48]. This study reported a method detection limit (MDL) of 0.08 μg/dL and had a detection frequency of 100% in a sample of 42 pregnant women exposed to high levels from artisanal and small-scale gold mining (ASGM) activities in Tanzania [48]. As noted by Parsons et al. [18], this study was especially impressive for its direct comparison of venous blood and DBS sample values and for its high level of agreement (R = 0.969) [48]. The study included both field and laboratory filter blanks to account for potential contamination, and reported field contamination about twice as high as laboratory contamination (0.02 μg/dL versus 0.009 μg/dL) [48], which is less than previously reported contamination levels (between 0.082 and 0.189 μg/dL) [52, 62]. This method had excellent reliability (intraclass correlation was 0.99 for repeated analyses of samples conducted on different days) [48]. DBS samples were stored at room temperature in a desiccator using trace metal-free Nalgene resealable plastic bags for 1–2 weeks prior to shipment to the laboratory [48]. The assay used full punch sizes of 8-mm diameter [48], which improved analytical sensitivity but limits the ability to perform further analyses using the same DBS samples due to finite sample quantity.
Rodríguez-Saldaña et al. validated an assay for quantifying Pb levels in DBS samples using total reflection X-ray fluorescence (TRXF) [46]. The LOD and LOQ for this assay were determined to be 0.28 and 0.69 µg/dL, respectively [46]. Using whole blood reference materials, this assay was determined to have a mean accuracy of 111.1% (97.0–129.7%) and a precision of 14.9% (<15% predefined acceptance criteria) [46]. Internal blanks were analyzed in 14% of the samples, and background Pb levels were essentially negligible [46]. This finding corroborates the low contamination levels reported by Nyanza et al. [48]; however, Funk et al. reported a median of 0.57 µg/dL [53] and geometric mean of 0.189 µg/dL [52] Pb in filter paper blanks. In the study by Rodríguez-Saldaña et al., there was a high level of agreement between TRXF-measured DBS values and venous blood values measured by ICP-MS as assessed by Bland-Altman analyses when applied to a low-exposure group (41 university students) and a relatively high-exposure group (40 electronic waste workers) [46]. Only 7.5% of the samples from the low-exposure group fell below the detection limit, while no samples were below the detection limit in the high-exposure group [46]. In addition, blank filter papers were analyzed from the high-exposure group, since these were collected from a contaminated field site, and no significant field contamination was found [46].
Specht et al. utilized energy-dispersive X-ray fluorescence (EDXRF) to measure the concentration of Pb from 22 DBS samples [63]. Here, Pb levels showed excellent agreement between EDXRF and atomic absorption spectroscopy (R = 0.98) [63]. The major advantages of using this EDXRF approach include [1] essentially avoiding potential effects of hematocrit since it is a measurement of the whole blood spot and [2] since EDXRF is a non-destructive process, DBS samples can be saved for further analyses [63]. The LOD of this method was 1.7 μg/dL blood [63], which is significantly higher than the reported detection limits of Rodríguez-Saldaña et al. [46] and other methods. However, increasing the power of the EDXRF system and employing longer measurement times (e.g., >30 min) may further decrease the detection limits and improve analytical sensitivity and precision in future studies [63].
Mercury
DBS assays for measuring mercury continue to evolve and there are several recent assays developed for ICP-MS [48, 52, 53], gas chromatography-cold vapor atomic fluorescence spectrometry (GC-CVAFS) [47, 64], and direct Hg analysis [49].
In addition to pre-treating filter paper cards to remove contamination, as discussed previously, Funk et al. demonstrated that the correlation between Hg in archived NDBS and paired filter blanks was significant (R = 0.44), suggesting that pair-wise blank subtractions may improve NDBS estimates [53]. However, in a subsequent study, which compared matched venous blood in trace metal-free vacutainers to prospectively collected DBS samples, it was determined that the use of filter paper blanks for background subtraction at the individual level does not work well for quantifying low levels of environmental exposure [52]. In addition, Funk et al. added gold to amalgamate Hg during blood extraction to provide higher extraction efficiency and prevent Hg loss throughout the analytical process [52]. Hg carryover between samples can also be avoided by introducing a wash step consisting of 5% HNO3 and 0.5% HCl solution between each ICP-MS run [52]. Nelson et al. also developed an ICP-MS assay for measuring total Hg (T-Hg) in DBS samples and reported an MDL for T-Hg of 0.7 µg/L, compared to 0.3 µg/L for cord blood, in a small cohort (n = 48) of urban Minnesota mothers and infants [51]. Because of this higher MDL, Hg exposure was detected in only 38% of NDBS samples compared to 62% of matched cord blood samples [51]. While T-Hg measurements in NDBS samples were highly correlated with matched cord blood samples, NDBS measurements were approximately 15% lower, on average [51].
The DBS assay developed by Nyanza et al. measured T-Hg using ICP-MS and had an MDL for T-Hg was 0.012 µg/L [48]. While using blank filter paper subtraction does not work well with low environmental exposures levels [52], performing blank subtractions in DBS samples collected from a high Hg exposure group may yield better results (although Hg contamination of field and laboratory blanks were quite low in this study, with a mean of 0.006 and 0.003 µg/L, respectively) [48]. After performing blank subtractions, Hg measurements in DBS were highly correlated with venous blood values and were found to be highly reliable with high intraclass correlations and repeatability between duplicate samples [48]. The correlation between DBS and venous blood measurements for Hg was higher than in previously reported studies [52], which the authors suggest could be due to previous studies mixing nitric acid (HNO3) and hydrochloric acid (HCl) during the digestion process [48].
Basu et al. developed and validated a highly-sensitive method using GC-CVAFS to quantify methyl mercury (Me-Hg) in NDBS samples [64]. This method had significantly improved analytical sensitivity and precision (LOQ of 0.3 µg/L) and was applied in a relatively large sample of NDBS samples from the Michigan BioTrust cohort (n = 675) [64]. Although NDBS measurements were not matched with cord blood data, the DBS values were within the expected range compared to other studies [64]. Hematocrit was investigated by Nelson et al. [51], in which no effects were found, but these analyses were limited by a small sample size [51].
Santa-Rios et al. [47] expanded on this DBS assay [64] to measure Me-Hg and inorganic Hg (I-Hg) in DBS samples using GC-CVAFS. This assay had an excellent agreement between DBS (capillary blood) and paired venous whole blood measures (R2 = 0.80), reported an MDL of 0.3 µg/L, and used a controlled sample volume (40 µL) in whole DBS spots to minimize potential hematocrit effects [47]. Moreover, Me-Hg measurements in DBS were found to be relatively stable for a 1-year storage period under room temperature conditions [47]. Of note, the previous assay developed by Basu et al. [64] used smaller sample volume requirements (estimated 3.1 µL) and achieved similar sensitivity and precision for detecting Me-Hg. Overall, these studies validated the use of DBS for Me-Hg quantification [47, 64], but quantification of I-Hg using this method had inadequate assay detection limits and requires further development [47]. It is worth noting that when analyzing Me-Hg using ICP-MS, chromatographic separation is required, which adds potential complexity to DBS analyses.
An assay has also recently been developed and validated for quantifying T-Hg by Direct Hg analysis based on atomic absorption spectroscopy and used three discs of 0.5 inches (~60 µL blood) [49]. This study demonstrated analyte stability in pre-cleaned glass tubes at 4 weeks and at elevated temperatures (40 °C) [49]. There was a high agreement between venous blood and DBS sample values, and the reported LOD and LOQ were 0.14 and 0.28 μg/L, respectively [49]. In addition, this study investigated the effects of different storage conditions on Hg stability in DBS samples, demonstrating that pre-cleaned glass tubes may be preferred over plastic bags for storing DBS samples for Hg analyses and that samples are stable for at least 4 weeks at both room temperature and at 40 °C [49].
Cadmium
DBS assays for measuring Cd have been limited by inadequate assay detection limits and varying degrees of background contamination of filter cards. Chaudhuri et al. used 6.35-mm punches (~11.5 µL blood) to quantify Cd in archived NDBS samples [62]. However, this study demonstrated high background contamination of filter paper cards, which made it difficult to produce reliable results. For example, DBS samples spiked with 0.62 μg/L of cadmium yielded a 53% recovery after performing blank subtractions [62]. Recovery rates were improved (87%) at higher DBS concentrations [62]. The authors concluded that more research was needed into methods development for this element, and additional experimentation investigating stability across time and storage conditions was not performed [62]. Langer et al. reported median background Cd contamination between 0.02 and 0.14 ng/spot across different lots [65]. This study was able to detect Cd in 100% of DBS samples (n = 150) at a median concentration of 0.24 ng/spot [65]. However, using different statistical correction methods in a smaller subset of samples (n = 15) resulted in Cd being detected in 0% of DBS samples [65]. This finding was somewhat unexplainable, although higher median Cd concentrations were found in adjacent filter blanks for samples detected only by the first statistical correction method used [65].
Funk et al. measured Cd concentrations of 0.2 ppb (0.2 μg/L) in NDBS samples after performing paired filter paper blank subtractions [53]. Cd was detectable in 67% of samples [53]. The correlation between Cd found in filter paper blanks and NDBS samples was significant (R = 0.60), suggesting that paired blank subtractions may improve estimates [53]. When filter paper cards were pretreated to remove contamination, NDBS and venous blood Cd values were highly agreeable (R2 = 0.94) [52]. Nyanza et al. developed a DBS assay for measuring Cd and, importantly, applied it to a high-exposure group [48]. This study found relatively insignificant levels of Cd in field filter blanks (mean = 0.0011 µg/L) and laboratory filter blanks (mean = 0.001 µg/L) [48]. The MDL was determined to be 0.004 µg/L and all DBS samples (n = 44) were above the detection limit [48]. The geometric mean DBS value was 0.361 µg/L (compared to 0.387 µg/L venous blood), indicating both high agreement with gold standard and a relatively high level of exposure among the study sample [48].
Arsenic
Blood is not a commonly used matrix for measuring As exposure due to its short residence time in the body [66]. Urine is a more commonly used sampling medium to measure As exposure [67, 68]. The assays developed by Funk et al. were the only methods developed to quantify As in DBS samples [52, 53]. The levels of filter paper blank contamination with As were low for most samples; however, spikes in values were observed in a minority of samples, suggesting possible heterogenous contamination of filter paper [52, 53]. In this study, 82% of the NDBS samples (n = 49) analyzed were below the detection limit [53]. Concentrations of As were undetectable in all filter paper blanks [53]. Therefore, pair-wise subtractions of filter paper blanks were not deemed necessary for studies interested in only As exposures [53]. Future work should increase the analytical sensitivity and precision of As quantification to reduce the number of non-detectable DBS samples [53].
Guidance
Pre-treating filter paper cards to remove trace element contamination prior to blood collection may improve assay performance [52]. Although inherent contamination in filter paper may be low [48], it is not consistent across lots of filter paper and contamination occurring before, during, and after blood collection may be much higher. Accounting for contamination by performing field and laboratory blank subtractions may be reasonable for relatively high-exposure groups [48]. Contamination of filter paper cards during manufacturing, collection, processing, and storing may be problematic for Pb and Cd, and possibly for As, but is less of a concern for Hg [51,52,53, 62, 65]. Future work should verify the low levels of contamination in filter paper blanks for Pb, Cd, and As reported by Nyanza et al. [48] and Rodríguez-Saldaña et al. [46]. Hg contamination may be introduced at higher storage temperatures depending on the storage container used [49]. As mentioned by Basu et al. [64], future work should address variations in blood spot volumes, perhaps by normalizing other blood constituents, such as potassium levels. Punching near the edge of blood spots may also minimize variation in blood spreading across the card [64].
According to US NHANES (2011–2018) biomonitoring data, the 50th percentile for blood lead levels is 0.46–0.64 μg/dL and the 90th percentile is 0.93–1.34 μg/dL among children ages 6–11 [37]. Therefore, the assays developed by Nyanza et al. (ICP-MS) [48] and Rodríguez-Saldaña et al. (TXRF) [46] have adequate analytical sensitivity and precision to detect and quantify these levels of lead exposure, with detection limits of 0.08 and 0.28 μg/dL, respectively. In contrast, the DBS methods developed by Specht et al. (EDXRF) [63] (detection limit of 1.7 μg/dL) will need further development to adequately characterize lead exposures in the general population. However, this assay has the major benefit of being non-destructive.
For biomonitoring of T-Hg and Me-Hg in the general population, current DBS assays similarly appear to have sufficient detection limits to characterize exposures. For example, using US NHANES (2011–2018) biomonitoring data, Me-Hg concentrations were 0.39–0.48 µg/L (50th percentile) and 2.23–2.81 µg/L (90th percentile) [37]. Therefore, Basu et al. [64] and Santa-Rios et al. [47] (using GC-CVAFS) report sufficient Me-Hg detection limits of ~0.3 µg/L. Similarly, blood T-Hg concentrations in the population were 0.58–0.64 µg/L (50th percentile) and 2.52–2.87 µg/L (90th percentile) compared to detection limits of 0.012 µg/L reported by Nyanza et al. [48] (ICP-MS) and 0.14 µg/L reported by Schweizer et al. [49] (Direct Hg analysis).
Although the MDL (0.004 µg/L) reported by Nyanza et al. [48] is sufficient to characterize exposures to Cd in the general US population (50th percentile: 0.22–0.25 µg/L, 90th percentile: 0.81–0.96 µg/L) [37], more research is needed to verify these detection limits given varying levels of accuracy, precision, and sensitivity in prior DBS assays [52, 53, 62, 65]. Similarly, more work is needed to sufficiently quantify As in populations with no known exposures.
Applications
In a cross-sectional study with a total of 1056 participants (part of the ongoing Mining and Health prospective longitudinal study), Nyanza et al. used DBS sampling to demonstrate that blood T-Hg levels in pregnant women were elevated in those who lived in ASGM communities, compared to a non-ASGM cohort, in Northern Tanzania (50th percentiles: 1.2 versus 0.66 µg/L and 75th percentiles: 1.86 versus 1.2 µg/L) [14]. Spot urine samples were used instead of DBS to estimate As exposure [14]. These findings were later extended to show that elevated blood T-Hg in DBS samples among pregnant women in ASGM communities were significantly associated with stillbirths and visible congenital anomalies [15]. In this same cohort, Nyanza et al. analyzed the associations between T-Hg, T-Pb, and T-Cd measured in maternal DBS samples (collected during weeks 16–27 of pregnancy) and neurodevelopmental outcomes in infants at 6 and 12 months of age [16]. These analyses included 439 mother–infant pairs, since they excluded maternal–infant pairs previously determined to have adverse birth outcomes [15] or lost to follow-up. The results demonstrated that high prenatal exposure to T-Hg was associated with neurodevelopmental and language impairments [16]. While prenatal exposures to high levels of Pb or As were not by themselves associated with neurodevelopmental impairments, prenatal co-exposure to high levels of T-Hg with elevated levels of Pb or As was associated with impairments in neurodevelopment, suggesting synergistic or additive effects [16].
Santa-Rios et al. extended their assay to measure both I-Hg and Me-Hg in DBS samples collected from ASGM and nearby Columbian communities using a cross-sectional study design (n = 35) [69]. T-Hg was measured from urine samples, which has been previously validated in exposure assessments [69]. The study used both field and laboratory blanks to account for potential contamination. In this study, only one and four samples were below the previously reported [47] detection limits for Me-Hg and I-Hg, respectively [69]. Field blanks had estimated contamination levels of ~0.07 and ~1.16 µg/L for Me-Hg and I-Hg, respectively [69]. Laboratory blanks had estimated contamination levels of ~0.15 and 1.77 µg/L for Me-Hg and I-Hg, respectively [69]. Me-Hg (%) speciation ranged from 5 to 100%, suggesting that future studies should continue to speciate T-Hg to more clearly identify sources of Hg exposure [69].
Santa-Rios et al. also extended their assay [47] to measure Me-Hg in DBS samples collected from electronic waste workers (n = 20) in Ghana [70]. DBS samples and venous blood were collected from the same study participants. DBS samples were also artificially created in the laboratory using collected venous blood samples. T-Hg was measured in venous blood samples. Only one sample fell below the MDL for Me-Hg [70]. There was excellent agreement between Me-Hg values measured in field-collected DBS samples, artificially created DBS samples, and gold standard venous blood samples [70]. Average Me-Hg concentrations were ~0.84 µg/L and Me-Hg speciation was 61% [70]. Me-Hg contamination of field blanks was low [70], corroborating prior studies.
Overall, quality and performance parameters for both application studies conducted by Santa-Rios et al. [69, 70] confirmed that their previously developed DBS methods [47] for measuring Me-Hg meet high-quality standards and are ready for deployment in larger-scale field- and population-based studies, including in contaminated field settings. However, future field-based studies should continue to report background contamination levels by using laboratory and field blanks.
In two studies by Sen et al. DBS sampling was applied to measure early-life exposure to Pb and associated epigenetic alterations [71, 72]. These studies used 3-mm punches and ICP-MS analyses for measuring blood Pb levels in DBS samples [71, 72]. DNA was isolated from the same DBS samples to characterize epigenetic profiles [72]. This group also analyzed associations between a mother’s archived NDBS and the child’s NDBS (collected from the Michigan Neonatal Biobank) to demonstrate that maternal Pb exposure during pregnancy can result in epigenetic alterations in grandchildren (i.e., multigenerational) [71]. Another study similarly used archived NDBS samples from Michigan to demonstrate that elevated newborn exposure to Pb was associated with greater epigenetic alterations, most prominently in pathways related to neurodevelopment [73]. This study used 3-mm DBS punches and reported an MDL of 0.7 µg/L [73]. Out of 129 samples, 21 were below the MDL [73]. The researchers highlight the unique utility of archived NDBS and prospectively collected DBS samples on accelerating the science of environmental epigenetics [73].
Industrial chemicals
Endocrine-disrupting chemicals and persistent organic pollutants
EDCs during the early stages of development can disrupt normal developmental patterns and may have low-dose and non-monotonic effects [74]. EDC exposure is associated with altered reproductive function, thyroid disruption, increased incidence of hormone-related cancers, abnormal growth patterns, neurodevelopmental disorders, and weakened immune systems [75,76,77]. EDCs include synthetic chemicals used as industrial solvents/lubricants, plastics, pesticides, and pharmaceutical agents [78]. Bisphenol A (BPA) can be found in consumer food and beverage products due to leaching from tinned containers [79]. BPA has been extensively studied and has been found in breast milk, amniotic fluid, and placental tissue [79]. BPA, a xenoestrogen, may have a role in reproductive cancers and fertility issues [79]. BPA has been phased out from most consumer containers and has been banned from infant products [80]. However, the safety profile of bisphenol analogs used as a replacement for BPA has not been well characterized [80]. Perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA), considered per- and polyfluoroalkyl substances (PFASs), are two other EDCs that have been extensively studied. PFOS and PFOA have recently become chemicals of interest after being found in drinking water in communities across the US [81].
POPs are chemicals that persist for long periods in the environment and can accumulate vertically in the food chain due to their ability to remain in adipose tissue [82, 83]. POPs include polychlorinated biphenyls (PCBs), PFASs, polybrominated diphenyl ethers (PBDEs), and organochlorine pesticides [84]. Several POPs, such as PCBs and PFASs, are also considered to be EDCs. PCBs have been associated with cancer and immune, reproductive, nervous system, endocrine system, and metabolic dysfunction [83, 85]. Although policy regulation has led to a decrease in exposure to chlorinated POPs among the general population, exposure to brominated POPs remains widespread [83]. Human exposure to POPs occurs primarily via the consumption of fatty animal-based foods [83]. Biomagnification can lead to human exposure several orders of magnitude greater than levels found in the environment, while its storage in adipose tissue leads to chronic endogenous exposure throughout the lifespan as it is continuously released from adipose tissue [83]. The persistent nature of POPs and their associated health effects make measuring and reducing exposure, especially among infants and children, a key public health concern.
Overview
A total of eight studies (five published reports) were primarily related to methods development and validation for measuring exposures to EDCs/POPs in DBS samples. Of these, four reports were from the US [86,87,88,89] and one was from Norway [90]. Three studies applied these methods to measure analytes in archived NDBS samples [86, 88, 89]. Two studies compared paired venous blood values to DBS measurements [87, 90].
Methods development
Barr et al. recently reviewed several DBS assays for measuring EDCs and POPs from a laboratory-based perspective, and suggested future considerations for improving the methods and reliability of DBS sampling for measuring these exposure biomarkers [17]. Here, we highlight the most well-developed and validated assays and their applications to population-based studies.
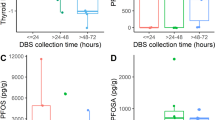
Ma et al. developed and validated methods for quantifying EDCs in DBS samples utilizing high-performance liquid chromatography (HPLC) and tandem MS to detect PFOS, PFOA, and BPA in 16-mm NDBS samples containing approximately 50 μL of blood [86]. Recovery rates from spiked samples were 79 and 92% for PFOS and PFOA, respectively, while BPA had a recovery rate of 39% [86]. Background levels of PFOS and PFOA were of minimal concern with trace amounts, 0.01 and 0.1 μg/L, respectively, found in filter paper blanks [86]. This contamination was thought to be from the reagents used and not from the filter paper itself. However, background levels of BPA in filter paper may be significant (0.5–0.8 μg/L) and should be taken into consideration [86]. PFOS had the lowest LOD at 0.03 μg/L (LOQ 0.1 μg/L), followed by PFOA at 0.05 μg/L (LOQ 0.2 μg/L), and BPA at 0.3 μg/L (1.0 μg/L) [86].
The method was applied to 192 NDBS samples from infants born in New York between 2008 and 2011 [86]. PFOS and PFOA were detected in 100% of samples analyzed with concentrations ranging from 0.27 to 6.46 μg/L and 0.21 to 4.35 μg/L, respectively [86]. Serum reference ranges among adolescents (ages 12–19) in the US (NHANES 2011–2018) were 2.60–4.11 μg/L (50th percentile) and 11.5–15.7 μg/L (90th percentile) for PFOS and 1.17–1.74 μg/L (50th percentile) and 2.07–2.93 μg/L (90th percentile) for PFOA [37]. BPA was found in 86% of samples at concentrations ranging from 0.2 to 35 ng/mL [86]. Field blanks were used to demonstrate that there was little contamination introduced during collection, storage, and shipping [86].
Poothong et al. developed a reliable method to measure a range of PFASs in human 3-mm punch DBS blood samples (~3.3 μL blood) from 59 Norwegian adults using an online solid phase extraction, ultra-high-performance liquid chromatography with tandem mass spectrometry (online SPE-UHPLC-MS/MS) quantification method [90]. For gold standard comparisons, 10 punches were used (~33 μL blood) and compared to whole DBS spots (~50 μL blood). These analyses demonstrated strong agreement between finger-prick DBS and venous whole blood samples (R = 0.72) [90]. The reported MDLs ranged from 0.008 to 0.3 μg/L, which were comparable to Ma et al. [91]. The study also did not find any significant effects of hematocrit on PFAS measurements [90]. Of the 25 PFASs measured in paired DBS and whole blood samples, only seven (perfluorohexane sulfonate (PFhxS), PFOS, PFOA, perfluorononanoic acid (PFNA), PFDA, PFUnDA, and perfluorooctane sulfonamide (PFOSA)) had satisfactory detection frequencies (>85%) and were used in further statistical analyses [90].
Batterman and Chernyak used GC-MS to measure 11 compounds including PCBs, PBDEs, and persistent pesticides in adult DBS samples [87]. The study found strong agreement between 50 μL DBS and whole blood samples from six volunteers [87]. Furthermore, sample integrity remained high in storage extending up to 1 year when samples were stored at refrigerated or frozen temperatures [87]. However, when stored at room temperature, sample integrity was high for up to 1 month [87]. Kato et al. also demonstrated the stability of several POPs in NDBS samples when stored at 37 °C for 61 days [88]. Batterman and Chernyak reported consistent background contamination of several POPs in DBS samples [87]. This contamination was confirmed to originate from the blank filter paper and not from the extraction or sample processing methods [87]. No additional contamination was observed as a function of storage time [87].
Applications
Spliethoff et al. used HPLC for temporal biomonitoring of PFOS, PFOSA, PFHxS, PFOA, and PFNA in 110 pooled composite DBS samples representing 2640 infants from New York State between 1997 and 2007 [92]. All analytes were detected in ≥90% of specimens and concentrations of PFOS, PFOSA, PFHxS, and PFOA decreased significantly after the year 2000, coinciding with the phasing out of PFOS production in the United States [92]. These methods were validated using spiked venous blood samples from adult volunteers [92]. Recoveries ranged from 60 to 112%, suggesting a slight bias toward lower values overall [92]. Field blanks were used to measure and adjust for background contamination present in the filter paper [92]. This study demonstrated the validity and efficacy of using pooled DBS sampling for temporal biomonitoring.
In two separate studies, Ma et al. used gas chromatography-high-resolution mass spectrometry for temporal biomonitoring by measuring exposure to POPs in 51 blood spot composites from 1224 newborns [91, 93]. The mean whole blood concentration of PCBs in Upstate New York newborn blood samples was found to be 1.06 ng/mL between 1997 and 2011, with a significant decrease between 1997 and 2001 and no significant reduction thereafter [91]. Ma et al. also observed mean concentrations of 0.128 ng/mL for PBDE congener brominated diphenyl ethers (BDE)-47, 0.040 ng/mL for BDE-99, and 0.012 ng/mL for BDE-100 [93]. Both studies used pooled blood spot composites resulting in a total estimated blood volume of 322 μL per sample [93]. The methodology was validated using spiked DBS samples at 0.2 and 2 ng/mL for each target compound [91, 93]. The PBDE congener recoveries ranged between 53.7 and 79.0% at the 0.2 ng/mL concentration and from 73.0 to 85.7% at the 2 ng/mL concentration. Consequently, PCB recoveries ranged between 51.8 and 102% at the 0.2 ng/mL concentration and from 89.2 to 114% at the 2 ng/mL concentration.
Several studies have applied the validated assay [86] developed by Ma et al. to measure concentrations of PFOS, PFOA, and BPA in archived NDBS samples collected from the Upstate KIDS Study (New York). Bell at el. measured PFOS, PFOA, and BPA in 3111 samples from singleton and twin infants and their relationship with infant health outcomes [94]. The study found that PFOS and PFOA levels were above detectable limits in >99% of samples and in 90% of samples for BPA [94]. The study observed no significant associations between PFAS and birth size controlling for plurality of birth, while BPA was negatively associated with birth size in twins [94]. In another analysis of the same NDBS data (n = 3111), Yeung et al. analyzed the association between newborn exposure to these EDCs and early childhood growth patterns, including weight gain and obesity rates. PFOS and PFOA values were highly correlated (R > 0.75) in NDBS samples from related twins; however, the association was lower for BPA (R = 0.23) [95]. The study suggested that newborn exposure to BPA may occur through extended hospital stays in the neonatal intensive care unit [95]. BPA measured in NDBS samples may therefore represent postnatal exposures (e.g., from medical devices) as opposed to prenatal exposures [95].
In the same study population and NDBS data, Ghassabian et al. assessed the relationship between PFOS, PFOA, and BPA and children’s behavior at 7 years [96]. In this analysis, 100% of specimens had detectable levels of PFOS and PFOA while BPA was detected in 86% of the specimens [96]. The differences in detection frequencies can be attributed to the smaller sample size used (n = 788 or 918 depending on the analysis). The study concluded that higher PFOS levels were associated with increased odds of behavioral difficulties, while increased PFOA was associated with difficulties in prosocial behaviors [96]. Neonatal BPA levels measured in NDBS, on the other hand, were not clearly associated with increased behavioral difficulties [96]. Another analysis of data from the Upstate KIDS Study found higher concentrations of some POPs associated with a small increased risk for gestational age and birth weight [97]. This study also demonstrated the potential utility of pooling DBS samples for increasing assay detection limits [97]. Most recently, Robinson et al. analyzed NDBS data (n = 597) from the Upstate KIDS Study for associations between PFOS and PFOA levels and epigentic alterations [98]. DNA was extracted from the NDBS samples using three discs of 0.5 inches [98]. Gross et al. also recently used NDBS samples to investigate the association between neonatal exposures to POPs and overweight status in a nested case–control study including a low-income Hispanic urban population [99]. Overall, these studies support the feasibility and utility of EDC quantification using residual NDBS samples.
Other environmental exposure biomarkers
Due to space limitations, we have not discussed here DBS assays to measure environmental exposures to benzene [100], fipronil (insecticide) [101], parabens [102], and acrylamide [103]. However, these assays are included in Table 1.
Discussion
In this review and guide for using DBS sampling in population-based research, we provide a summary of DBS assays that have been developed and validated for measuring exposure biomarkers for investigators that are collecting, or planning to collect, DBS samples to investigate environmental causes of disease. The use of DBS sampling to estimate environmental exposures to chemical toxicants provides a simple and non-invasive means for obtaining blood samples in population-based studies, which is particularly well suited for field-based studies conducted in low-resource settings and in large cohort studies involving infants and children. Recent improvements in analytical sensitivities have vastly reduced blood volume requirements allowing for accurate detection and quantification of an array of exposure biomarkers. Together, these advancements provide extensive opportunities for investigating links between environmental exposures and adverse health outcomes.
High-performing DBS methods have been developed, validated, and applied for measuring exposures to ETS (cotinine), trace elements (e.g., Pb and Hg), and several important EDCs and POPs. In addition, DBS assays tend to show high correlations with gold standard venous blood assays for many exposure biomarkers, including cotinine, lead, total mercury, methyl mercury, and several EDCs and POPs. As a result, DBS sampling may be an attractive option in epidemiological studies measuring these biomarkers when venous blood collection is not feasible. In addition, DBS sampling presents a unique opportunity to advance environmental epigenetics, especially among hard-to-reach populations [71,72,73].
However, uncertainties remain regarding background contamination levels in filter paper, especially for Pb, As, Cd, and BPA. Additional work is also needed to improve the MDLs (i.e., sensitivity and precision) of assays for measuring As, Cd, and BPA before their widespread use in large-scale population-based studies. Future method development studies should ensure consistent evaluation and reporting of key quality-control assay parameters, including precision, reliability, accuracy/recovery, sensitivity, stability, and detection frequencies [9, 17], to accelerate improvements in analytical performance and facilitate comparisons between assays. Studies applying previously developed DBS methods to population-based studies should continue to report quality assurance parameters and should perform method and field blank subtractions to facilitate DBS sampling as a reliable tool for advancing public health and environmental epidemiology. In addition, the DBS assays discussed here have not yet been reliably reproduced across different laboratories, which would be a major next step in validation [54].
The implementation of DBS sampling in low- and middle-income countries may be enhanced by existing public health infrastructure that collects DBS samples for other purposes, such as for the monitoring of antiretroviral treatment among HIV-positive patients (i.e., viral load measurements), surveillance of HIV drug resistance, expansion of early infant diagnosis of HIV programs, or for malaria diagnostic testing [48, 69, 70, 104,105,106,107,108,109]. Another promising avenue of future research is the prospect of enabling study participants to self-collect DBS samples [10, 11]. With the persistence of the COVID-19 pandemic, widespread collection of DBS samples are being incorporated into community- and hospital-based seroprevalence studies, which use DBS sampling to detect the levels of SARS-CoV-2 IgG antibodies [110,111,112]. Future research may use residual DBS samples collected for the purposes of seroprevalence studies for measuring exposure biomarkers among subpopulations of interest.
In addition, because of the COVID-19 pandemic, many existing environmental health cohort studies have been disrupted and have not been able to collect blood samples from study participants as planned (e.g., ECHO cohorts). As an alternative method for measuring exposure biomarkers with well-developed and validated assays, self-collection of DBS samples may be a feasible method for continuing to obtain blood samples during potentially critical developmental periods for study participants. However, contamination remains a significant issue for many target analytes in DBS samples. Therefore, contamination may be a concern with the self-collection of DBS samples by untrained study participants, which will need to be addressed in future investigations.
Change history
02 November 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41370-022-00488-9
References
Angerer J, Ewers U, Wilhelm M. Human biomonitoring: state of the art. Int J Hyg Environ Health. 2007;210:201–28.
Sobus JR, DeWoskin RS, Tan YM, Pleil JD, Phillips MB, George BJ, et al. Uses of NHANES biomarker data for chemical risk assessment: trends, challenges, and opportunities. Environ Health Perspect. 2015;123:919–27.
Edwards SW, Preston RJ. Systems biology and mode of action based risk assessment. Toxicological Sci. 2008;106:312–8.
Pleil JD, Sheldon LS. Adapting concepts from systems biology to develop systems exposure event networks for exposure science research. Biomarkers. 2011;16:99–105.
Smolders R, Schramm KW, Nickmilder M, Schoeters G. Applicability of non-invasively collected matrices for human biomonitoring. Environ Health. 2009;8:8.
Sobus JR, Tan YM, Pleil JD, Sheldon LS. A biomonitoring framework to support exposure and risk assessments. Sci Total Environ. 2011;409:4875–84.
Esteban M, Castano A. Non-invasive matrices in human biomonitoring: a review. Environ Int. 2009;35:438–49.
Wallace MA, Kormos TM, Pleil JD. Blood-borne biomarkers and bioindicators for linking exposure to health effects in environmental health science. J Toxicol Environ Health B Crit Rev. 2016;19:380–409.
McDade TW. Development and validation of assay protocols for use with dried blood spot samples. Am J Hum Biol. 2014;26:1–9.
Allen AM, Lundeen K, Murphy SE, Spector L, Harlow BL. Web-delivered multimedia training materials for the self-collection of dried blood spots: a formative project. JMIR Form Res. 2018;2:e11025.
Sullivan PS, Sailey C, Guest JL, Guarner J, Kelley C, Siegler AJ, et al. Detection of SARS-CoV-2 RNA and antibodies in diverse samples: protocol to validate the sufficiency of provider-observed, home-collected blood, saliva, and oropharyngeal samples. JMIR Public Health Surveill. 2020;6:e19054.
Forrest C, Blackwell C, Camargo C Jr. Advancing the science of children’s positive health in the NIH Environmental influences on Child Health Outcomes (ECHO) Research Program. J Pediatr. 2018;196:298–300.
Buckley JP, Barrett ES, Beamer PI, Bennett DH, Bloom MS, Fennell TR, et al. Opportunities for evaluating chemical exposures and child health in the United States: the Environmental influences on Child Health Outcomes (ECHO) Program. J Expo Sci Environ Epidemiol. 2020;30:397–419.
Nyanza EC, Bernier FP, Manyama M, Hatfield J, Martin JW, Dewey D. Maternal exposure to arsenic and mercury in small-scale gold mining areas of Northern Tanzania. Environ Res. 2019;173:432–42.
Nyanza EC, Dewey D, Manyama M, Martin JW, Hatfield J, Bernier FP. Maternal exposure to arsenic and mercury and associated risk of adverse birth outcomes in small-scale gold mining communities in Northern Tanzania. Environ Int. 2020;137:105450.
Nyanza E, Bernier F, Martin J, Manyama M, Hatfield J, Dewey D. Effects of prenatal exposure and co-exposure to metallic or metalloid elements on early infant neurodevelopmental outcomes in areas with small-scale gold mining activities in Northern Tanzania. Environ Int. 2021;149:106104.
Barr DB, Kannan K, Cui Y, Merrill L, Petrick LM, Meeker JD, et al. The use of dried blood spots for characterizing children’s exposure to organic environmental chemicals. Environ Res. 2021;195:110796.
Parsons P, Galusha A, Cui Y, Faustman E, Falman J, Meeker J, et al. A critical review of the analysis of dried blood spots for characterizing human exposure to inorganic targets using methods based on analytical atomic spectrometry. R Soc Chem. 2020;35:2092–112.
Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–43.
Naylor S, Kajbaf M, Lamb JH, Jahanshahi M, Gorrod JW. An evaluation of tandem mass spectrometry in drug metabolism studies. Biol Mass Spectrom. 1993;22:388–94.
Health Resources and Services Administration. Recommended Uniform Screening Panel. 2020. Available from: https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/index.html
Therrell BL, Padilla CD, Loeber JG, Kneisser I, Saadallah A, Borrajo GJ, et al. Current status of newborn screening worldwide: 2015. Semin Perinatol. 2015;39:171–87.
Angerer J, Mannschreck C, Gündel J. Biological monitoring and biochemical effect monitoring of exposure to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health. 1997;70:365–77.
Wagner M, Tonoli D, Varesio E, Hopfgartner G. The use of mass spectrometry to analyze dried blood spots. Mass Spectrom Rev. 2016;35:361–438.
Olshan AF. Meeting report: the use of newborn blood spots in environmental research: opportunities and challenges. Environ Health Perspect. 2007;115:1767–79.
DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113:1007–15.
Pope CA, Burnett RT, Turner MC, Cohen A, Krewski D, Jerrett M, et al. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect. 2011;119:1616–21.
Murphy SE, Wickham KM, Lindgren BR, Spector LG, Joseph A. Cotinine and trans 3’-hydroxycotinine in dried blood spots as biomarkers of tobacco exposure and nicotine metabolism. J Expo Sci Environ Epidemiol. 2013;23:513–8.
Tretzel L, Thomas A, Piper T, Hedeland M, Geyer H, Schänzer W, et al. Fully automated determination of nicotine and its major metabolites in whole blood by means of a DBS online-SPE LC-HR-MS/MS approach for sports drug testing. J Pharm Biomed Anal. 2016;123:132–40.
Spector LG, Hecht SS, Ognjanovic S, Carmella SG, Ross JA. Detection of cotinine in newborn dried blood spots. Cancer Epidemiol Biomark Prev. 2007;16:1902–5.
Yang J, Pearl M, Jacob P, DeLorenze GN, Benowitz NL, Yu L, et al. Levels of cotinine in dried blood specimens from newborns as a biomarker of maternal smoking close to the time of delivery. Am J Epidemiol. 2013;178:1648–54.
Searles Nielsen S, Dills RL, Glass M, Mueller BA. Accuracy of prenatal smoking data from Washington State birth certificates in a population-based sample with cotinine measurements. Ann Epidemiol. 2014;24:236–9.
Sosnoff CS, Bernert JT. Analysis of cotinine in dried blood spots by LC APCI tandem mass spectrometry. Clin Chim Acta. 2008;388:228–9.
Ladror D, Pitt B, Funk W. Quantification of cotinine in dried blood spots as a biomarker of exposure to tobacco smoke. Biomarkers 2018;23:44–50.
Joseph A, Spector L, Wickham K, Janis G, Winickoff J, Lindgren B, et al. Biomarker evidence of tobacco smoke exposure in children participating in lead screening. Am J Public Health. 2013;103:e54–9.
Spector LG, Murphy SE, Wickham KM, Lindgren B, Joseph AM. Prenatal tobacco exposure and cotinine in newborn dried blood spots. Pediatrics. 2014;133:e1632–8.
Biomonitoring Data Tables for Environmental Chemicals [dataset]. Centers for Disease Control and Prevention. 2022. Available from: https://www.cdc.gov/exposurereport/data_tables.html
American Heart Association. Structural Racism & Tobacco. 2020. Available from: https://www.heart.org/-/media/Files/About-Us/Policy-Research/Fact-Sheets/Tobacco-and-Clean-Air/Structural-Racism-and-Tobacco-Fact-Sheet.pdf
Cosselman KE, Navas-Acien A, Kaufman JD. Environmental factors in cardiovascular disease. Nat Rev Cardiol. 2015;12:627–42.
Kim HC, Jang TW, Chae HJ, Choi WJ, Ha MN, Ye BJ, et al. Evaluation and management of lead exposure. Ann Occup Environ Med. 2015;27:30.
National Center for Environmental Health, Division of Environmental Health Science and Practice. Blood Lead Reference Value. 2021. Available from: https://www.cdc.gov/nceh/lead/data/blood-lead-reference-value.htm
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N. Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol. 2013;47:4967–83.
Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, et al. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect. 2012;120:799–806.
Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, et al. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect. 2013;121:295–302.
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A. The effects of cadmium toxicity. Int J Environ Res Public Health. 2020;17:3782.
Rodríguez-Saldaña V, Fobil J, Basu N. Lead (Pb) exposure assessment in dried blood spots using total reflection X-ray fluorescence (TXRF). Environ Res. 2021;198:110444.
Santa-Rios A, Barst BD, Basu N. Mercury speciation in whole blood and dried blood spots from capillary and venous sources. Anal Chem. 2020;92:3605–12.
Nyanza EC, Dewey D, Bernier F, Manyama M, Hatfield J, Martin JW. Validation of dried blood spots for maternal biomonitoring of nonessential elements in an artisanal and small-scale gold mining area of Tanzania. Environ Toxicol Chem. 2019;38:1285–93.
Schweizer AK, Kabesch M, Quartucci C, Bose-O’Reilly S, Rakete S. Implementation of mercury biomonitoring in German adults using dried blood spot sampling in combination with direct mercury analysis. Environ Monit Assess. 2021;193:488.
Archer NP, Bradford CM, Klein DM, Barnes J, Smith LJ, Villanacci JF. Relationship between prenatal lead exposure and infant blood lead levels. Matern Child Health J. 2012;16:1518–24.
Nelson JW, Edhlund BL, Johnson J, Rosebush CE, Holmquist ZS, Swan SH, et al. Assessing a new method for measuring fetal exposure to mercury: newborn bloodspots. Int J Environ Res Public Health. 2016;13:692.
Funk WE, Pleil JD, Sauter DJ, McDade TW, Holl JL. Use of dried blood spots for estimating children’s exposures to heavy metals in epidemiological research. J Environ Anal Toxicol. 2015;S7:002.
Funk WE, McGee JK, Olshan AF, Ghio AJ. Quantification of arsenic, lead, mercury and cadmium in newborn dried blood spots. Biomarkers. 2013;18:174–7.
Stanton NV, Maney JM, Jones R. Evaluation of filter paper blood lead methods: results of a pilot proficiency testing program. Clin Chem. 1999;45:2229–35.
Wang ST, Demshar HP. Determination of blood lead in dried blood-spot specimens by Zeeman-effect background corrected atomic absorption spectrometry. Analyst. 1992;117:959–61.
Verebey K, Eng Y, Davidow B, Ramon A. Rapid, sensitive micro blood lead analysis: a mass screening technique for lead poisoning. J Anal Toxicol. 1991;15:237–40.
Cernik AA, Sayers MH. Determination of lead in capillary blood using a paper punched disc atomic absorption technique. Application to the supervision of lead workers. Br J Ind Med. 1971;28:392–8.
Cernik AA. Determination of blood lead using a 4.0 mm paper punched disc carbon sampling cup technique. Br J Ind Med. 1974;31:239–44.
Carter GF. The paper punched disc technique for lead in blood samples with abnormal haemoglobin values. Br J Ind Med. 1978;35:235–40.
Srivuthana K, Yee HY, Bhambhani K, Elton RM, Simpson PM, Kauffman RE. A new filter paper method to measure capillary blood lead level in children. Arch Pediatr Adolesc Med. 1996;150:498–502.
Yee HY, Holtrop TG. An improved capillary blood-filter paper-graphite furnace atomic absorption spectrometric method for lead screening. J Anal Toxicol. 1997;21:142–8.
Chaudhuri SN, Butala SJ, Ball RW, Braniff CT, Consortium RMB. Pilot study for utilization of dried blood spots for screening of lead, mercury and cadmium in newborns. J Expo Sci Environ Epidemiol. 2009;19:298–316.
Specht AJ, Obrycki JF, Mazumdar M, Weisskopf MG. Feasibility of lead exposure assessment in blood spots using energy-dispersive X-ray fluorescence. Environ Sci Technol. 2021;55:5050–5.
Basu N, Eng JWL, Perkins M, Santa-Rios A, Martincevic G, Carlson K, et al. Development and application of a novel method to characterize methylmercury exposure in newborns using dried blood spots. Environ Res. 2017;159:276–82.
Langer EK, Johnson KJ, Shafer MM, Gorski P, Overdier J, Musselman J, et al. Characterization of the elemental composition of newborn blood spots using sector-field inductively coupled plasma-mass spectrometry. J Expo Sci Environ Epidemiol. 2011;21:355–64.
Subcommittee on Arsenic in Drinking Water. In: National Research Council, editor. Arsenic in drinking water. Washington, DC: National Academy Press; 1999.
Verdon CP, Caldwell KL, Fresquez MR, Jones RL. Determination of seven arsenic compounds in urine by HPLC-ICP-DRC-MS: a CDC population biomonitoring method. Anal Bioanal Chem. 2009;393:939–47.
Howe CG, Farzan SF, Garcia E, Jursa T, Iyer R, Berhane K, et al. Arsenic and birth outcomes in a predominately lower income Hispanic pregnancy cohort in Los Angeles. Environ Res. 2020;184:109294.
Santa-Rios A, Barst BD, Tejeda-Benitez L, Palacios-Torres Y, Baumgartner J, Basu N. Dried blood spots to characterize mercury speciation and exposure in a Colombian artisanal and small-scale gold mining community. Chemosphere. 2021;266:129001.
Santa-Rios A, Fobil J, Basu N. Methylmercury measurements in dried blood spots from electronic waste workers sampled from Agbogbloshie, Ghana. Environ Toxicol Chem. 2021;40:2183–8.
Sen A, Heredia N, Senut MC, Land S, Hollocher K, Lu X, et al. Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Sci Rep. 2015;5:14466.
Sen A, Heredia N, Senut MC, Hess M, Land S, Qu W, et al. Early life lead exposure causes gender-specific changes in the DNA methylation profile of DNA extracted from dried blood spots. Epigenomics. 2015;7:379–93.
Montrose L, Goodrich JM, Morishita M, Kochmanski J, Klaver Z, Cavalcante R, et al. Neonatal lead (Pb) exposure and DNA methylation profiles in dried bloodspots. Int J Environ Res Public Health. 2020;17:6775.
Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee DH, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455.
Schug TT, Blawas AM, Gray K, Heindel JJ, Lawler CP. Elucidating the links between endocrine disruptors and neurodevelopment. Endocrinology. 2015;156:1941–51.
Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127:204–15.
Sifakis S, Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Human exposure to endocrine disrupting chemicals: effects on the male and female reproductive systems. Environ Toxicol Pharm. 2017;51:56–70.
Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342.
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol. 2007;24:139–77.
Chen D, Kannan K, Tan H, Zheng Z, Feng YL, Wu Y, et al. Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity—a review. Environ Sci Technol. 2016;50:5438–53.
Cordner A, De La Rosa VY, Schaider LA, Rudel RA, Richter L, Brown P. Guideline levels for PFOA and PFOS in drinking water: the role of scientific uncertainty, risk assessment decisions, and social factors. J Expo Sci Environ Epidemiol. 2019;29:157–71.
Guo W, Pan B, Sakkiah S, Yavas G, Ge W, Zou W, et al. Persistent organic pollutants in food: contamination sources, health effects and detection methods. Int J Environ Res Public Health. 2019;16:4361.
Lee DH. Persistent organic pollutants and obesity-related metabolic dysfunction: focusing on type 2 diabetes. Epidemiol Health. 2012;34:e2012002.
Fry K, Power MC. Persistent organic pollutants and mortality in the United States, NHANES 1999-2011. Environ Health. 2017;16:105.
Alharbi O, Basheer A, Khattab R, Ali I. Health and environmental effects of persistent organic pollutants. J Mol Liq. 2018;263:442–53.
Ma W, Kannan K, Wu Q, Bell EM, Druschel CM, Caggana M, et al. Analysis of polyfluoroalkyl substances and bisphenol A in dried blood spots by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. 2013;405:4127–38.
Batterman S, Chernyak S. Performance and storage integrity of dried blood spots for PCB, BFR and pesticide measurements. Sci Total Environ. 2014;494–495:252–60.
Kato K, Wanigatunga AA, Needham LL, Calafat AM. Analysis of blood spots for polyfluoroalkyl chemicals. Anal Chim Acta. 2009;656:51–5.
Burse VW, DeGuzman MR, Korver MP, Najam AR, Williams CC, Hannon WH, et al. Preliminary investigation of the use of dried-blood spots for the assessment of in utero exposure to environmental pollutants. Biochem Mol Med. 1997;61:236–9.
Poothong S, Papadopoulou E, Lundanes E, Padilla-Sánchez JA, Thomsen C, Haug LS. Dried blood spots for reliable biomonitoring of poly- and perfluoroalkyl substances (PFASs). Sci Total Environ. 2019;655:1420–6.
Ma WL, Gao C, Bell EM, Druschel CM, Caggana M, Aldous KM, et al. Analysis of polychlorinated biphenyls and organochlorine pesticides in archived dried blood spots and its application to track temporal trends of environmental chemicals in newborns. Environ Res. 2014;133:204–10.
Spliethoff HM, Tao L, Shaver SM, Aldous KM, Pass KA, Kannan K, et al. Use of newborn screening program blood spots for exposure assessment: declining levels of perluorinated compounds in New York State infants. Environ Sci Technol. 2008;42:5361–7.
Ma WL, Yun S, Bell EM, Druschel CM, Caggana M, Aldous KM, et al. Temporal trends of polybrominated diphenyl ethers (PBDEs) in the blood of newborns from New York State during 1997 through 2011: analysis of dried blood spots from the newborn screening program. Environ Sci Technol. 2013;47:8015–21.
Bell EM, Yeung EH, Ma W, Kannan K, Sundaram R, Smarr MM, et al. Concentrations of endocrine disrupting chemicals in newborn blood spots and infant outcomes in the upstate KIDS study. Environ Int. 2018;121:232–9.
Yeung EH, Bell EM, Sundaram R, Ghassabian A, Ma W, Kannan K, et al. Examining endocrine disruptors measured in newborn dried blood spots and early childhood growth in a prospective cohort. Obesity (Silver Spring) 2019;27:145–51.
Ghassabian A, Bell EM, Ma WL, Sundaram R, Kannan K, Buck Louis GM, et al. Concentrations of perfluoroalkyl substances and bisphenol A in newborn dried blood spots and the association with child behavior. Environ Pollut. 2018;243:1629–36.
Bell GA, Perkins N, Buck Louis GM, Kannan K, Bell EM, Gao C, et al. Exposure to persistent organic pollutants and birth characteristics: the Upstate KIDS Study. Epidemiology. 2019;30:S94–S100.
Robinson SL, Zeng X, Guan W, Sundaram R, Mendola P, Putnick DL, et al. Perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS) and DNA methylation in newborn dried blood spots in the Upstate KIDS cohort. Environ Res. 2021;194:110668.
Gross RS, Ghassabian A, Vandyousefi S, Messito MJ, Gao C, Kannan K, et al. Persistent organic pollutants exposure in newborn dried blood spots and infant weight status: a case-control study of low-income Hispanic mother-infant pairs. Environ Pollut. 2020;267:115427.
Funk WE, Waidyanatha S, Chaing SH, Rappaport SM. Hemoglobin adducts of benzene oxide in neonatal and adult dried blood spots. Cancer Epidemiol Biomark Prev. 2008;17:1896–901.
Raju KS, Taneja I, Rashid M, Sonkar AK, Wahajuddin M, Singh SP. DBS-platform for biomonitoring and toxicokinetics of toxicants: proof of concept using LC-MS/MS analysis of fipronil and its metabolites in blood. Sci Rep. 2016;6:22447.
Mulla H, Yakkundi S, McElnay J, Lutsar I, Metsvaht T, Varendi H, et al. An observational study of blood concentrations and kinetics of methyl- and propyl-parabens in neonates. Pharm Res. 2015;32:1084–93.
Starlin Z, Harahap Y, S Sitepu E. Method validation of acrylamide in dried blood spot by liquid chromatography-tandem mass spectrometry. Pak J Biol Sci. 2020;23:1321–31.
Mishra V, Vaessen M, Boerma JT, Arnold F, Way A, Barrere B, et al. HIV testing in national population-based surveys: experience from the Demographic and Health Surveys. Bull World Health Organ. 2006;84:537–45.
Phillips A, Shroufi A, Vojnov L, Cohn J, Roberts T, Ellman T, et al. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature. 2015;528:S68–76.
Bertagnolio S, Parkin NT, Jordan M, Brooks J, García-Lerma JG. Dried blood spots for HIV-1 drug resistance and viral load testing: a review of current knowledge and WHO efforts for global HIV drug resistance surveillance. AIDS Rev. 2010;12:195–208.
Parkin NT. Measurement of HIV-1 viral load for drug resistance surveillance using dried blood spots: literature review and modeling of contribution of DNA and RNA. AIDS Rev. 2014;16:160–71.
Roberts T, Cohn J, Bonner K, Hargreaves S. Scale-up of routine viral load testing in resource-poor settings: current and future implementation challenges. Clin Infect Dis. 2016;62:1043–8.
Ciaranello AL, Park JE, Ramirez-Avila L, Freedberg KA, Walensky RP, Leroy V. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med. 2011;9:59.
McDade TW, McNally EM, D’Aquila R, Mustanski B, Miller A, Vaught LA, et al. Enzyme immunoassay for SARS-CoV-2 antibodies in dried blood spot samples: a minimally-invasive approach to facilitate community- and population-based screening. medRxiv [Preprint]. Available from: https://www.medrxiv.org/content/10.1101/2020.04.28.20081844v1.
McDade TW, McNally EM, Zelikovich AS, D'Aquila R, Mustanski B, Miller A, et al. High seroprevalence for SARS-CoV-2 among household members of essential workers detected using dried blood spot assay. PLOS ONE. 2020;15:e0237833.
Moat SJ, Zelek WM, Carne E, Ponsford MJ, Bramhall K, Jones S, et al. Development of a high-throughput SARS-CoV-2 antibody testing pathway using dried blood spot specimens. Annals of Clinical Biochemistry. 2021;58:123–31.
Acknowledgements
The authors thank Patricia Smith, information specialist at the Galter Health Sciences Library at Northwestern University, for assistance and guidance in defining our initial publication search criteria. We also would like to acknowledge the excellent work of the reviewers who helped us improve the presentation of this manuscript. This publication was supported in part by the Office of The Director, National Institutes of Health under Award Number U24OD023319. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This work was supported by grant number R21ES026776 from the National Institutes of Environmental Health Sciences and the Environmental Influences on Child Health Outcomes (ECHO) program, Office of The Director, National Institutes of Health under Award Number U24OD023319, with cofunding from the Office of Behavioral and Social Sciences Research (OBSSR; Person-Reported Outcomes Core).
Author information
Authors and Affiliations
Contributions
TAJ drafted the manuscript. All authors revised the manuscript, provided key guidance, and approved the final manuscript. TAJ and WEF were involved in the conception of the manuscript. DAN and TAJ collaboratively developed the systematic search strategy. DAN performed a systematic literature search. TAJ, JSK, YB, RI, and NDM performed data extraction. TAJ, JSK, and YB developed the figures and tables.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jacobson, T.A., Kler, J.S., Bae, Y. et al. A state-of-the-science review and guide for measuring environmental exposure biomarkers in dried blood spots. J Expo Sci Environ Epidemiol 33, 505–523 (2023). https://doi.org/10.1038/s41370-022-00460-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41370-022-00460-7
Keywords
This article is cited by
-
Trace elements in hair or fingernail and gastroesophageal cancers: results from a population-based case-control study
Journal of Exposure Science & Environmental Epidemiology (2023)
-
Per- and polyfluoroalkyl substances (PFAS) and thyroid hormone measurements in dried blood spots and neonatal characteristics: a pilot study
Journal of Exposure Science & Environmental Epidemiology (2023)
-
Reliability and feasibility of home-based dried blood spot in therapeutic drug monitoring: a systematic review and meta-analysis
European Journal of Clinical Pharmacology (2023)
-
Advances in the Use of Residual Newborn Dried Blood Spots Within Environmental Epidemiology
Current Epidemiology Reports (2023)