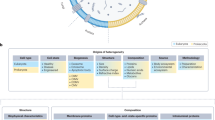
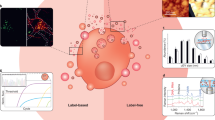
Abstract
Extracellular vesicles (EVs) represent small, membrane-enclosed particles that are derived from parent cells and are secreted into the extracellular space. Once secreted, EVs can then travel and communicate with nearby or distant cells. Due to their inherent stability and biocompatibility, these particles can effectively transfer RNAs, proteins, and chemicals/metabolites from parent cells to target cells, impacting cellular and pathological processes. EVs have been shown to respond to disease-causing agents and impact target cells. Given that disease-causing agents span environmental contaminants, pathogens, social stressors, drugs, and other agents, the translation of EV methods into public health is now a critical research gap. This paper reviews approaches to translate EVs into exposure science, toxicology, and public health applications, highlighting blood as an example due to its common use within clinical, epidemiological, and toxicological studies. Approaches are reviewed surrounding the isolation and characterization of EVs and molecular markers that can be used to inform EV cell-of-origin. Molecular cargo contained within EVs are then discussed, including an original analysis of blood EV data from Vesiclepedia. Methods to evaluate functional consequences and target tissues of EVs are also reviewed. Lastly, the expanded integration of these approaches into future public health applications is discussed, including the use of EVs as promising biomarkers of exposure, effect, and disease.
Impact statement
-
Extracellular vesicles (EVs) represent small, cell-derived structures consisting of molecules that can serve as biomarkers of exposure, effect, and disease.
-
This review lays a novel foundation for integrating EVs, a rapidly advancing molecular biological tool, into the field of public health research including epidemiological, toxicological, and clinical investigations.
-
This article represents an important advancement in public health and exposure science as it is among the first to translate EVs into this field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Yekula A, Muralidharan K, Kang KM, Wang L, Balaj L, Carter BS. From laboratory to clinic: Translation of extracellular vesicle based cancer biomarkers. Methods 2020;177:58–66.
Huang G, Lin G, Zhu Y, Duan W, Jin D. Emerging technologies for profiling extracellular vesicle heterogeneity. Lab Chip. 2020;20:2423–37.
Bazzan E, Tinè M, Casara A, Biondini D, Semenzato U, Cocconcelli E, et al. Critical Review of the Evolution of Extracellular Vesicles’ Knowledge: from 1946 to Today. Int J Mol Sci. 2021;22:6417.
Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189–97.
Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–88.
Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–39.
Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 1983;33:967–78.
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesic. 2018;7:1535750.
Hartjes TA, Mytnyk S, Jenster GW, van Steijn V, van Royen ME. Extracellular Vesicle Quantification and Characterization:common methods and emerging approaches. Bioengineering (Basel). 2019;6.
Vesiclepedia. A community compendium for extracellular vesicles 2021 [cited 2021 May 1]. http://www.microvesicles.org/.
Baxter AA. Stoking the fire: how dying cells propagate inflammatory signalling through extracellular vesicle trafficking. Int J Mol Sci. 2020;21.
Li X, Corbett AL, Taatizadeh E, Tasnim N, Little JP, Garnis C, et al. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019;3:011503.
Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of Exosome Composition. Cell 2019;177:428–45 e18.
Squadrito ML, Baer C, Burdet F, Maderna C, Gilfillan GD, Lyle R, et al. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep. 2014;8:1432–46.
Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5.
Cha DJ, Franklin JL, Dou Y, Liu Q, Higginbotham JN, Demory Beckler M, et al. KRAS-dependent sorting of miRNA to exosomes. Elife 2015;4:e07197.
Fu S, Zhang Y, Li Y, Luo L, Zhao Y, Yao Y. Extracellular vesicles in cardiovascular diseases. Cell Death Disco. 2020;6:68.
Kwok ZH, Wang C, Jin Y. Extracellular Vesicle Transportation and Uptake by Recipient Cells: a critical process to regulate human diseases. Processes (Basel). 2021;9.
Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27:172–88.
Xavier CPR, Caires HR, Barbosa MAG, Bergantim R, Guimaraes JE, Vasconcelos MH. The Role of Extracellular Vesicles in the Hallmarks of Cancer and Drug Resistance. Cells. 2020;9.
Freeman DW, Noren Hooten N, Eitan E, Green J, Mode NA, Bodogai M, et al. Altered Extracellular Vesicle Concentration, Cargo, and Function in Diabetes. Diabetes 2018;67:2377–88.
Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, et al. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS ONE. 2014;9:e113651.
Eichner NZM, Gilbertson NM, Gaitan JM, Heiston EM, Musante L, LaSalvia S, et al. Low cardiorespiratory fitness is associated with higher extracellular vesicle counts in obese adults. Physiol Rep. 2018;6:e13701.
Yang TT, Liu CG, Gao SC, Zhang Y, Wang PC. The Serum Exosome Derived MicroRNA-135a, -193b, and -384 Were Potential Alzheimer’s Disease Biomarkers. Biomed Environ Sci. 2018;31:87–96.
Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New Technologies for Analysis of Extracellular Vesicles. Chem Rev. 2018;118:1917–50.
Benjamin RJ, McLaughlin LS. Plasma components: properties, differences, and uses. Transfusion 2012;52:9S–19S.
Phillips W, Willms E, Hill AF. Understanding extracellular vesicle and nanoparticle heterogeneity: novel methods and considerations. Proteomics 2021;21:e2000118.
Breitman TR, He RY. Combinations of retinoic acid with either sodium butyrate, dimethyl sulfoxide, or hexamethylene bisacetamide synergistically induce differentiation of the human myeloid leukemia cell line HL60. Cancer Res. 1990;50:6268–73.
Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, et al. Methodological Guidelines to Study Extracellular Vesicles. Circ Res. 2017;120:1632–48.
Palviainen M, Saraswat M, Varga Z, Kitka D, Neuvonen M, Puhka M, et al. Extracellular vesicles from human plasma and serum are carriers of extravesicular cargo-Implications for biomarker discovery. PLoS ONE. 2020;15:e0236439.
Lacroix R, Judicone C, Mooberry M, Boucekine M, Key NS, Dignat-George F, et al. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2013.
Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2.
Chiam K, Mayne GC, Wang T, Watson DI, Irvine TS, Bright T, et al. Serum outperforms plasma in small extracellular vesicle microRNA biomarker studies of adenocarcinoma of the esophagus. World J Gastroenterol. 2020;26:2570–83.
Zarovni N, Corrado A, Guazzi P, Zocco D, Lari E, Radano G, et al. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 2015;87:46–58.
Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics 2017;7:789–804.
Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomed. 2020;15:6917–34.
Atha DH, Ingham KC. Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J Biol Chem. 1981;256:12108–17.
Yuana Y, Koning RI, Kuil ME, Rensen PC, Koster AJ, Bertina RM, et al. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J Extracell Vesicles. 2013;2.
Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 2011;7:780–8.
Welsh JA, Holloway JA, Wilkinson JS, Englyst NA. Extracellular Vesicle Flow Cytometry Analysis and Standardization. Front Cell Dev Biol. 2017;5:78.
Vogel R, Willmott G, Kozak D, Roberts GS, Anderson W, Groenewegen L, et al. Quantitative sizing of nano/microparticles with a tunable elastomeric pore sensor. Anal Chem. 2011;83:3499–506.
Akers JC, Ramakrishnan V, Nolan JP, Duggan E, Fu CC, Hochberg FH, et al. Comparative Analysis of Technologies for Quantifying Extracellular Vesicles (EVs) in Clinical Cerebrospinal Fluids (CSF). PLoS ONE. 2016;11:e0149866.
Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip. 2014;14:1891–900.
Jorgensen M, Baek R, Pedersen S, Sondergaard EK, Kristensen SR, Varming K. Extracellular Vesicle (EV) Array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J Extracell Vesicles. 2013;2.
Jorgensen MM, Baek R, Varming K. Potentials and capabilities of the Extracellular Vesicle (EV) Array. J Extracell Vesicles. 2015;4:26048.
Koliha N, Wiencek Y, Heider U, Jungst C, Kladt N, Krauthauser S, et al. A novel multiplex bead-based platform highlights the diversity of extracellular vesicles. J Extracell Vesicles. 2016;5:29975.
Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113:E968–77.
Martin-Jaular L, Nevo N, Schessner JP, Tkach M, Jouve M, Dingli F, et al. Unbiased proteomic profiling of host cell extracellular vesicle composition and dynamics upon HIV-1 infection. EMBO J. 2021:e105492.
Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30–41.
Skotland T, Sagini K, Sandvig K, Llorente A. An emerging focus on lipids in extracellular vesicles. Adv Drug Deliv Rev. 2020;159:308–21.
Guduric-Fuchs J, O’Connor A, Camp B, O’Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genom. 2012;13:357.
Li Y, He X, Li Q, Lai H, Zhang H, Hu Z, et al. EV-origin: enumerating the tissue-cellular origin of circulating extracellular vesicles using exLR profile. Comput Struct Biotechnol J. 2020;18:2851–9.
Clark J, Avula V, Ring C, Eaves LA, Howard T, Santos HP, et al. Comparing the Predictivity of Human Placental Gene, microRNA, and CpG Methylation Signatures in Relation to Perinatal Outcomes. Toxicol Sci. 2021;183:269–84.
Ring C, Sipes NS, Hsieh JH, Carberry C, Koval LE, Klaren WD, et al. Predictive modeling of biological responses in the rat liver using in vitro Tox21 bioactivity: Benefits from high-throughput toxicokinetics. Comput Toxicol. 2021;18.
Lage K, Hansen NT, Karlberg EO, Eklund AC, Roque FS, Donahoe PK, et al. A large-scale analysis of tissue-specific pathology and gene expression of human disease genes and complexes. Proc Natl Acad Sci USA. 2008;105:20870–5.
Zavala J, Freedman AN, Szilagyi JT, Jaspers I, Wambaugh JF, Higuchi M, et al. New Approach Methods to Evaluate Health Risks of Air Pollutants: Critical Design Considerations for In Vitro Exposure Testing. Int J Environ Res Public Health. 2020;17.
Rager JE, Bangma J, Carberry C, Chao A, Grossman J, Lu K, et al. Review of the environmental prenatal exposome and its relationship to maternal and fetal health. Reprod Toxicol. 2020;98:1–12.
Clark J, Rager JE. Chapter 1. Epigenetics: An overview of CpG methylation, chromatin remodeling, and regulatory/non-coding RNAs. In: Fry RC, editor. Environmental Epigenetics in Toxicology and Public Health. London, United Kingdom: Academic Press, an imprint of Elsevier; 2020. p. 3–32.
Fry RC, Bangma J, Szilagyi J, Rager JE. Developing novel in vitro methods for the risk assessment of developmental and placental toxicants in the environment. Toxicol Appl Pharm. 2019;378:114635.
Aristizabal-Henao JJ, Ahmadireskety A, Griffin EK, Da Silva BF, Bowden JA. Lipidomics and environmental toxicology: recent trend. Curr Opin Environ Sci Health. 2020;15:26–31.
Pathan M, Fonseka P, Chitti SV, Kang T, Sanwlani R, Van Deun J, et al. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47:D516–D9.
Vesiclepedia. Browse results for cell type/tissue: Plasma 2021 [cited 2021 May 15]. http://microvesicles.org/browse_results?org_name=&cont_type=&tissue=Plasma&gene_symbol=&ves_type=.
Kim J, Tan Z, Lubman DM. Exosome enrichment of human serum using multiple cycles of centrifugation. Electrophoresis 2015;36:2017–26.
LifeMapSciences. GeneCards THE HUMAN GENE DATABASE: MIR142 2021 [cited 2021 Nov 6]. https://www.genecards.org/cgi-bin/carddisp.pl?gene=MIR142&keywords=miR-142-3p.
LifeMapSciences. GeneCards THE HUMAN GENE DATABASE: MIR103A1 2021 [cited 2021 Nov 6]. https://www.genecards.org/cgi-bin/carddisp.pl?gene=MIR103A1.
LifeMapSciences. GeneCards THE HUMAN GENE DATABASE: MIRLET7A1 2021 [cited 2021 Nov 6]. https://www.genecards.org/cgi-bin/carddisp.pl?gene=MIRLET7A1&keywords=let-7a.
Endzelins E, Berger A, Melne V, Bajo-Santos C, Sobolevska K, Abols A, et al. Detection of circulating miRNAs: comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer. 2017;17:730.
Catalano M, O’Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J Extracell Vesicles. 2020;9:1703244.
Gong M, Yu B, Wang J, Wang Y, Liu M, Paul C, et al. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 2017;8:45200–12.
Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–30.
Im EJ, Lee CH, Moon PG, Rangaswamy GG, Lee B, Lee JM, et al. Sulfisoxazole inhibits the secretion of small extracellular vesicles by targeting the endothelin receptor A. Nat Commun. 2019;10:1387.
Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Investig. 2010;120:457–71.
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–79.
McCann JV, Bischoff SR, Zhang Y, Cowley DO, Sanchez-Gonzalez V, Daaboul GD, et al. Reporter mice for isolating and auditing cell type-specific extracellular vesicles in vivo. Genesis 2020;58:e23369.
Ryu AR, Kim DH, Kim E, Lee MY. The Potential Roles of Extracellular Vesicles in Cigarette Smoke-Associated Diseases. Oxid Med Cell Longev. 2018;2018:4692081.
Comfort N, Smith C, Chillrud S, Yang Q, Baccarelli A, Jack D. Extracellular Vesicles in Saliva as Biomarkers of Exposure and Effect: a feasibility pilot in the context of the New York City Biking and Breathing Study. Environ Epidemiol.2019;3:https://doi.org/10.1097/01.EE9.0000606548.23536.31.
Martin PJ, Heliot A, Tremolet G, Landkocz Y, Dewaele D, Cazier F, et al. Cellular response and extracellular vesicles characterization of human macrophages exposed to fine atmospheric particulate matter. Environ Pollut. 2019;254:112933.
Le Goff M, Lagadic-Gossmann D, Latour R, Podechard N, Grova N, Gauffre F, et al. PAHs increase the production of extracellular vesicles both in vitro in endothelial cells and in vivo in urines from rats. Environ Pollut. 2019;255:113171.
Hinzman CP, Baulch JE, Mehta KY, Girgis M, Bansal S, Gill K, et al. Plasma-derived extracellular vesicles yield predictive markers of cranial irradiation exposure in mice. Sci Rep. 2019;9:9460.
Fares J, Kashyap R, Zimmermann P. Syntenin: Key player in cancer exosome biogenesis and uptake? Cell Adh Migr. 2017;11:124–6.
Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–24.
Di Vizio D, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol. 2012;181:1573–84.
Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91.
Yan C, Hu J, Yang Y, Hu H, Zhou D, Ma M, et al. Plasma extracellular vesiclepackaged microRNAs as candidate diagnostic biomarkers for earlystage breast cancer. Mol Med Rep. 2019;20:3991–4002.
Szajnik M, Derbis M, Lach M, Patalas P, Michalak M, Drzewiecka H, et al. Exosomes in Plasma of Patients with Ovarian Carcinoma: Potential Biomarkers of Tumor Progression and Response to Therapy. Gynecol Obstet (Sunnyvale). 20133.
Baran J, Baj-Krzyworzeka M, Weglarczyk K, Szatanek R, Zembala M, Barbasz J, et al. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Cancer Immunol Immunother. 2010;59:841–50.
Kim HK, Song KS, Park YS, Kang YH, Lee YJ, Lee KR, et al. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer. 2003;39:184–91.
Minciacchi VR, Freeman MR, Di, Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51.
Kupper N, Huppertz B. The endogenous exposome of the pregnant mother: placental extracellular vesicles and their effect on the maternal system. Mol Aspects Med. 2021:100955.
O’Reilly D, Dorodnykh D, Avdeenko NV, Nekliudov NA, Garssen J, Elolimy AA, et al. Perspective: The Role of Human Breast-Milk Extracellular Vesicles in Child Health and Disease. Adv Nutr. 2021;12:59–70.
Merchant ML, Rood IM, Deegens JKJ, Klein JB. Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery. Nat Rev Nephrol. 2017;13:731–49.
UniProt. UniProtKB 2021 [cited 2021 May 15]. https://www.uniprot.org/uniprot/.
Laulagnier K, Javalet C, Hemming FJ, Chivet M, Lachenal G, Blot B, et al. Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell Mol Life Sci. 2018;75:757–73.
Liao Z, Jaular LM, Soueidi E, Jouve M, Muth DC, Schoyen TH, et al. Acetylcholinesterase is not a generic marker of extracellular vesicles. J Extracell Vesicles. 2019;8:1628592.
Wahlund CJE, Eklund A, Grunewald J, Gabrielsson S. Pulmonary Extracellular Vesicles as Mediators of Local and Systemic Inflammation. Front Cell Dev Biol. 2017;5:39.
Khalyfa A, Kheirandish-Gozal L. Gozal DExosome and Macrophage Crosstalk in Sleep-Disordered Breathing-Induced Metabolic Dysfunction. Int J Mol Sci. 2018;19.
Schierer S, Ostalecki C, Zinser E, Lamprecht R, Plosnita B, Stich L, et al. Extracellular vesicles from mature dendritic cells (DC) differentiate monocytes into immature DC. Life Sci Alliance. 2018;1:e201800093.
Hough KP, Deshane JS. Cutting edge approaches for rapid characterization of airway exosomes. Methods 2020;177:27–34.
Herrera Sanchez MB, Previdi S, Bruno S, Fonsato V, Deregibus MC, Kholia S, et al. Extracellular vesicles from human liver stem cells restore argininosuccinate synthase deficiency. Stem Cell Res Ther. 2017;8:176.
Chen W, Huang Y, Han J, Yu L, Li Y, Lu Z, et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64:831–40.
Oksvold MP, Kullmann A, Forfang L, Kierulf B, Li M, Brech A, et al. Expression of B-cell surface antigens in subpopulations of exosomes released from B-cell lymphoma cells. Clin Ther. 2014;36:847–62 e1.
Multia E, Tear CJY, Palviainen M, Siljander P, Riekkola ML. Fast isolation of highly specific population of platelet-derived extracellular vesicles from blood plasma by affinity monolithic column, immobilized with anti-human CD61 antibody. Anal Chim Acta. 2019;1091:160–8.
Muraoka S, Jedrychowski MP, Tatebe H, DeLeo AM, Ikezu S, Tokuda T, et al. Proteomic Profiling of Extracellular Vesicles Isolated From Cerebrospinal Fluid of Former National Football League Players at Risk for Chronic Traumatic Encephalopathy. Front Neurosci. 2019;13:1059.
Jiang L, Shen Y, Guo D, Yang D, Liu J, Fei X, et al. EpCAM-dependent extracellular vesicles from intestinal epithelial cells maintain intestinal tract immune balance. Nat Commun. 2016;7:13045.
Ravenhill BJ, Kanjee U, Ahouidi A, Nobre L, Williamson J, Goldberg JM, et al. Quantitative comparative analysis of human erythrocyte surface proteins between individuals from two genetically distinct populations. Commun Biol. 2019;2:350.
Choi H, Kim Y, Mirzaaghasi A, Heo J, Kim YN, Shin JH, et al. Exosome-based delivery of super-repressor IkappaBalpha relieves sepsis-associated organ damage and mortality. Sci Adv. 2020;6:eaaz6980.
Zhu Z, Shen Y, Chen Y, Shi H, Shi Y. The exosome of platelet endothelial cell adhesion molecule-1 (PECAM1) protein: a potential risking star in high blood pressure patients (HBPP. Medicine (Baltimore). 2021;100:e21370.
Miranda J, Paules C, Nair S, Lai A, Palma C, Scholz-Romero K, et al. Placental exosomes profile in maternal and fetal circulation in intrauterine growth restriction - Liquid biopsies to monitoring fetal growth. Placenta 2018;64:34–43.
Mu W, Provaznik J, Hackert T, Zoller M. Tspan8-Tumor Extracellular Vesicle-Induced Endothelial Cell and Fibroblast Remodeling Relies on the Target Cell-Selective Response. Cells. 2020;9.
Funding
This study was supported by grants from the National Institutes of Health (NIH) from the National Institute of Environmental Health Sciences (1R21ES031740, P42ES031007). Support was additionally provided through the Institute for Environmental Health Solutions (IEHS) at the University of North Carolina Gillings School of Global Public Health.
Author information
Authors and Affiliations
Contributions
CKC, DK, and JER were responsible for reviewing available literature and drafting review text. AP was responsible for extracting and analyzing extracellular vesicle molecular data. CKC and AP were responsible for manuscript visualizations. GJS and JER were responsible for reviewing overall content and providing feedback.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Carberry, C.K., Keshava, D., Payton, A. et al. Approaches to incorporate extracellular vesicles into exposure science, toxicology, and public health research. J Expo Sci Environ Epidemiol 32, 647–659 (2022). https://doi.org/10.1038/s41370-022-00417-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41370-022-00417-w