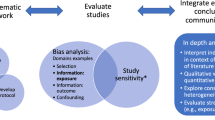
Abstract
Systematic review (SR) is a rigorous methodology applied to synthesize and evaluate a body of scientific evidence to answer a research or policy question. Effective use of systematic-review methodology enables use of research evidence by decision makers. In addition, as reliance on systematic reviews increases, the required standards for quality of evidence enhances the policy relevance of research. Authoritative guidance has been developed for use of SR to evaluate evidence in the fields of medicine, social science, environmental epidemiology, toxicology, as well as ecology and evolutionary biology. In these fields, SR is typically used to evaluate a cause–effect relationship, such as the effect of an intervention, procedure, therapy, or exposure on an outcome. However, SR is emerging to be a useful methodology to transparently review and integrate evidence for a wider range of scientifically informed decisions and actions across disciplines. As SR is being used more broadly, there is growing consensus for developing resources, guidelines, ontologies, and technology to make SR more efficient and transparent, especially for handling large amounts of diverse data being generated across multiple scientific disciplines. In this article, we advocate for advancing SR methodology as a best practice in the field of exposure science to synthesize exposure evidence and enhance the value of exposure studies. We discuss available standards and tools that can be applied and extended by exposure scientists and highlight early examples of SRs being developed to address exposure research questions. Finally, we invite the exposure science community to engage in further development of standards and guidance to grow application of SR in this field and expand the opportunities for exposure science to inform environment and public health decision making.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ellwood K, Trumbo P, Kavanaugh C. How the US Food and Drug Administration evaluates the scientific evidence for health claims. Nutr Rev. 2010;68:114–21. https://www.ncbi.nlm.nih.gov/pubmed/20137056. https://doi.org/10.1111/j.1753-4887.2009.00267.x.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Meta-analyses of randomized controlled clinical trials to evaluate the safety of human drugs or biological products guidance for industry. Draft guidance. Silver Spring, MD: Office of Communications, Division of Drug Information Center for Drug Evaluation and Research, Food and Drug Administration; 2018. https://www.fda.gov/media/117976/download.
U.S. DHHS, Agency for Healthcare Research and Quality. Improving access to and usability of systematic review data for health systems guidelines development. Rockville, MD: Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services; 2019. https://effectivehealthcare.ahrq.gov/products/systematic-review-data/methods-report. Accessed 9 Nov 2019.
Birnbaum L, Thayer K, Bucher J, Wolfe M. Implementing systematic review at the national toxicology program: status and next steps. Environ Health Perspect. 2013;121:A108–9. https://doi.org/10.1289/ehp.130671.
NIEHS, National Toxicology Program. Handbook for conducting a literature-based health assessment using OHAT approach for systematic review and evidence integration. https://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookmarch2019_508.pdf. Accessed 9 May 2019.
National Academies of Sciences, Engineering, and Medicine. Progress toward transforming the integrated risk information system (IRIS) program: a 2018 evaluation. Washington, DC: The National Academies Press; 2018. https://doi.org/10.17226/25086.
US EPA. Office of Chemical Safety and Pollution Prevention. Application of systematic review in TSCA risk evaluations. EPA Document# 740-P1-8001. 2018. https://www.epa.gov/sites/production/files/2018-06/documents/final_application_of_sr_in_tsca_05-31-18.pdf. Accessed 13 Jan 2020.
European Centre for Disease Prevention and Control. Systematic reviews. European Centre for Disease Prevention and Control; 2020. https://www.ecdc.europa.eu/en/all-topics-z/scientific-advice/systematic-reviews.
European Food Safety Authority. Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA J. 2010;8:1637. https://doi.org/10.2903/j.efsa.2010.1637.https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2010.1637.
Institute of Medicine. Finding what works in health care: standards for systematic reviews. Washington, DC: The National Academies Press; 2011. https://doi.org/10.17226/13059. Accessed 8 Nov 2018.
Stewart L, Moher D, Shekelle P. Why prospective registration of systematic reviews makes sense. Syst Rev. 2012;1:7. https://doi.org/10.1186/2046-4053-1-7.
National Institute for Health Research (NIHR). PROSPERO: international prospective register of systematic reviews. https://www.crd.york.ac.uk/prospero/. Accessed 20 Nov 2019.
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf Libraries J. 2009;26:91–108.
Dickersin K, Chalmers F. Thomas C Chalmers (1917–1995): a pioneer of randomized clinical trials and systematic reviews. JLL Bulletin: Commentaries on the history of treatment evaluation. 2014. https://www.jameslindlibrary.org/articles/thomas-c-chalmers-1917-1995/.
Clarke M, Chalmers I. Reflections on the history of systematic reviews. BMJ Evid-Based Med. 2018;23:121–2.
Woodruff TJ, Sutton P. The navigation guide systematic review methodology: a rigorous and transparent method for translating environmental health science into better health outcomes. Environ Health Perspect. 2014;122:1007–14.
Whaley P, Halsall C, Agerstrand M, Aiassa E, Benford D, Bilotta G, et al. Implementing systematic review techniques in chemical risk assessment: challenges, opportunities and recommendations. Environ Int. 2015; 92–93:556–64. https://www.sciencedirect.com/science/article/pii/S0160412015300866.
Radke, Galizia A, Thayer KA, Cooper GS. Phthalate exposure and metabolic effects: a systematic review of the human epidemiological evidence. Environment Int. 2019;132. https://doi.org/10.1016/j.envint.2019.04.040.
Mandrioli D, Schlünssen V, Adam B, Cohen RA, Chen W, Colosio C, et al. WHO/ILO work-related burden of disease and injury: protocols for systematic reviews of occupational exposure to dusts and/or fibres and of the effect of occupational exposure to dusts and/or fibres on pneumoconiosis. Environ Int. 2018;119:174–85.
VanNoy, BN, J Lam, AR Zota. Breastfeeding as a predictor of serum concentrations of per and polyfluorinated alkyl substances in reproductive-aged women and young children: a rapid systematic review. Curr Environ Health Rep. 2018. https://doi.org/10.1007/s40572-018-0194-z.
Frank JJ, Poulakos AG, Tornero-Velez R, Xue J. Systematic review and meta-analyses of lead (Pb) concentrations in environmental media (soil, dust, water, food, and air) reported in the United States from 1996 to 2016. Sci Total Environ 2019;694:133489. https://doi.org/10.1016/j.scitotenv.2019.07.295.
Cano-Sancho G, Ploteau S, Mattaa K, Adoamnei E, Buck Louis G, Mendiola J, et al. Human epidemiological evidence about the associations between exposure to organochlorine chemicals and endometriosis: systematic review and meta-analysis. Environ Int. 2019;123:209–23. https://doi.org/10.1016/j.envint.2018.11.065.
David A Savitz, Gregory A Wellenius, Thomas A Trikalinos. The problem with mechanistic risk of bias assessments in evidence synthesis of observational studies and a practical alternative: assessing the impact of specific sources of potential bias. Am J Epidemiol. 2019;188:1581–5. https://doi.org/10.1093/aje/kwz131.
Morgan RL, Whaley P, Thayer KA, Schunemann HJ. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121:1027–1031.
McKeon JMM, McKeon PO. PICO: a hot topic in evidence-based practice. Int J Athl Ther Train. 2015;20:1–3.
Singh JA, Sperling J, Buchbinder R, McMaken K. Surgery for shoulder osteoarthritis: a Cochrane systematic review. J Rheumatol. 2011;38:598–605.
Gates S. Review of methodology of quantitative reviews using meta‐analysis in ecology. J Anim Ecol. 2002;71:547–557.
Haddaway NR, Bilotta GS. Systematic reviews: separating fact from fiction. Environ Int. 2016;92:578–84.
Vreeman RC, Carroll AE. A systematic review of school-based interventions to prevent bullying. Arch Pediatr Adolesc Med. 2007;161:78–88.
Cimino AM, Boyles AL, Thayer KA, Perry MJ. Effects of neonicotinoid pesticide exposure on human health: a systematic review. Environ Health Perspect. 2016;125:155–62.
U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: a report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006.
National Research Council 2014. Review of EPA’s Integrated risk information system (IRIS) process. Washington, DC: The National Academies Press. https://doi.org/10.17226/18764.
Deziel NC, Freeman LE, Graubard BI, Jones RR, Hoppin JA, Thomas K, et al. Relative contributions of agricultural drift, para-occupational, and residential use exposure pathways to house dust pesticide concentrations: meta-regression of published data. Environ Health Perspect. 2017;125:296–305.
Locke SJ, Deziel NC, Koh DH, Graubard BI, Purdue MP, Friesen MC. Evaluating predictors of lead exposure for activities disturbing materials painted with or containing lead using historic published data from U.S. workplaces. Am J Ind Med. 2017;60:189–97.
Whetzel PL, Noy NF, Shah NH, Alexander PR, Nyulas C, Tudorache T, et al. BioPortal: enhanced functionality via new Web services from the National Center for Biomedical Ontology to access and use ontologies in software applications. Nucleic Acids Res. 2011;39:W541–5. https://doi.org/10.1093/nar/gkr469.
Ives C, Campia I, Wang R, Wittwehr C, Edwards S. Creating a structured AOP knowledgebase via ontology-based annotations. Applied In Vitro Toxicol. 2017;3:298–311. https://doi.org/10.1089/aivt.2017.0017.
Mattingly CJ, McKone TE, Callahan MA, Blake JA, Hubal EAC. Providing the missing link: the exposure science ontology ExO. Environ Sci Technol. 2012;46:3046–53.
Meyer DE, Bailin SC, Vallero D, Egeghy PP, Liu SV, Cohen Hubal EA. Enhancing life cycle chemical exposure assessment through ontology modeling. Sci Total Environ. 2020;712:136263. https://doi.org/10.1016/j.scitotenv.2019.136263.
Howard BE, Phillips J, Miller K, Tandon A, Mav D, Shah MR, et al. “SWIFT-review: a text-mining workbench for systematic review”. Syst Rev. 2016;5:87. https://doi.org/10.1186/s13643-016-0263-z.
U.S. EPA. Integrated science assessment (ISA) for particulate matter (Final Report, 2019). Washington, DC: U.S. Environmental Protection Agency; 2019. EPA/600/R-19/188.
Miller K, Howard BE, Phillips J, Shah M, Mav D, Shah R. “SWIFT-active screener: reducing literature screening effort through machine learning for systematic reviews”. New Orleans, Louisiana: Society of Toxicology Meeting; 2016.
Howard BE, Miller K, Phillips J, Thayer K, Shah R. “Evaluation of the literature prioritization capabilities of SWIFT-review, a tool for conducting systematic reviews of environmental health questions”. New Orleans, Louisiana: Society of Toxicology Meeting; 2016.
Wolffe T, Whaley P, Halsall C, Rooney A, Walker V. Systematic evidence maps as a novel tool to support evidence-based decision-making in chemicals policy and risk management. Environ Int. 2019;130. https://www.sciencedirect.com/science/article/pii/S0160412019310323.
National Institute of Standards and Technology, Informatiom Technology Laboratory. Text analysis conference. Systematic Review Information Extraction (SRIE). 2018. https://tac.nist.gov/2018/SRIE/.
Acknowledgements
The U.S. EPA and Exposure Science in the 21st Century Federal Working Group gathered US Federal Agency experts for a summit in April 2019 to explore how systematic review is being used and could be applied to exposure sciences. The summit was chaired by authors MF and RN in collaboration with the EPA Systematic Review Community of Practice, whose co-chairs EL and Kristan Markey saw the timeliness of conversation around systematic review and exposure sciences. Many of the topics in this perspective stem from the presentations and conversations initiated at that event. The authors thank Ashlei Williams and Rachel Slover for formating this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cohen Hubal, E.A., Frank, J.J., Nachman, R. et al. Advancing systematic-review methodology in exposure science for environmental health decision making. J Expo Sci Environ Epidemiol 30, 906–916 (2020). https://doi.org/10.1038/s41370-020-0236-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41370-020-0236-0