Abstract
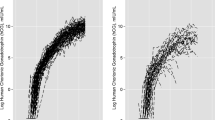
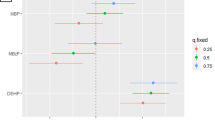
This study examines critical issues in accounting for urinary dilution in peri-implantation samples used to assess environmental exposures. Early pregnancy could impact creatinine excretion, which could bias biomarker measurement and interpretation when creatinine adjustment is used. We compared creatinine levels pre-implantation with levels soon after implantation at 3–6 weeks gestation. Using data and urine specimens from 145 women who conceived, we used linear mixed models to estimate the effect of pregnancy on creatinine concentrations. We also studied whether creatinine adjustment is biased when using pooled, within-person samples rather than averaging individually-adjusted results. For this, we grouped 2655 daily urinary estrogen metabolite and associated creatinine measures into 762 mathematically-constructed sample pools, and compared averaged individual measures with pooled measures using weighted kappa coefficients and t-tests. Urinary creatinine concentration declined an average of 14% (95% CI: −19, −11%) from pre- to post-implantation. While there was strong correlation between results based on the two creatinine adjustment methods, adjustment based on pooled specimens introduced a small 3% (95% CI: 2, 4%) underestimation of the analyte compared with averaging individually-adjusted samples. Postimplantation creatinine declines could introduce errors in biomonitoring results when comparing exposure measures from pre- and post-implantation. Though pooled creatinine adjustment underestimated adjusted analyte concentrations, the bias was small and agreement excellent between pooled and averaged individually-adjusted assessments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200.
MacPherson S, Arbuckle TE, Fisher M. Adjusting urinary chemical biomarkers for hydration status during pregnancy. J Expo Sci Environ Epidemiol. 2018;28:481–93.
Sauvé JF, Lévesque M, Huard M, Drolet D, Lavoué J, Tardif R, et al. Creatinine and specific gravity normalization in biological monitoring of occupational exposures. J Occup Environ Hyg. 2015;12:123–9.
Hays SM, Aylward LL, Blount BC. Variation in urinary flow rates according to demographic characteristics and body mass index in NHANES: potential confounding of associations between health outcomes and urinary biomarker concentrations. Environ Health Perspect. 2015;123:293–300.
Miller RC, Brindle E, Holman DJ, Shofer J, Klein NA, Soules MR, et al. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clin Chem. 2004;50:924–32.
Carrieri M, Trevisan A, Bartolucci GB. Adjustment to concentration-dilution of spot urine samples: correlation between specific gravity and creatinine. Int Arch Occup Environ Health. 2001;74:63–7.
Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54:615–27.
Davison JM, Noble MC. Serial changes in 24h creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. Br J Obstet Gynaecol. 1981;88:10–7.
Weaver VM, Kotchmar DJ, Fadrowski JJ, Silbergeld EK. Challenges for environmental epidemiology research: Are biomarker concentrations altered by kidney function or urine concentration adjustment? J Expo Sci Environ Epidemiol. 2016;26:1–8.
Aylward LL, Hays SM, Smolders R, Koch HM, Cocker J, Jones K, et al. Sources of variability in biomarker concentrations. J Toxicol Environ Heal - Part B Crit Rev. 2014;17:45–61.
O’Brien KM, Upson K, Cook NR, Weinberg CR. Environmental chemicals in urine and blood: Improving methods for creatinine and lipid adjustment. Environ Health Perspect. 2016;124:220–7.
Abduljalil K, Furness P, Johnson TN, Rostami-Hodjegan A, Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2012;51:365–96.
Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis. 2013;20:209–14.
Odutayo A, Hladunewich M. Obstetric nephrology: renal hemodynamic and metabolic physiology in normal pregnancy. Clin J Am Soc Nephrol. 2012;7(December):2073–80.
Baba Y, Furuta I, Zhai T, Ohkuchi A, Yamada T, Takahashi K, et al. Effect of urine creatinine level during pregnancy on dipstick test. J Obstet Gynaecol Res. 2017;43:967–73.
Schisterman EF, Vexler A. To pool or not to pool, from whether to when: applications of pooling to biospecimens subject to a limit of detection. Paediatr Perinat Epidemiol. 2008;22:486–96.
Perrier F, Giorgis-Allemand L, Slama R, Philippat C. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology 2016;27:378–88.
Weinberg CR, Shi M, O’Brien KM, Umbach DM. Adjustment for urinary creatinine or serum lipids for analytes assayed in pooled specimens. Epidemiology 2019;30:768–79.
Wilcox AJ, Weinberg CR, Wehmann RE, Armstrong EG, Canfield RE, Nisula BC. Measuring early pregnancy loss: laboratory and field methods. Fertil Steril. 1985;44:366–74.
Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N. Engl J Med. 1988;319:189–94.
Jukic AM, Calafat AM, McConnaughey DR, Longnecker MP, Hoppin JA, Weinberg CR, et al. Urinary Concentrations of Phthalate Metabolites and Bisphenol A and Associations with Follicular-Phase Length, Luteal-Phase Length, Fecundability, and Early Pregnancy Loss. Environ Health Perspect. 2016;124:321–8.
Baird DD, Weinberg CR, Wilcox AJ, McConnaughey DR, Musey PI. Using the ratio of urinary oestrogen and progesterone metabolites to estimate day of ovulation. Stat Med. 1991;10:255–66.
Baird DD, Weinberg CR, Zhou H, Kamel F, McConnaughey DR, Kesner JS, et al. Preimplantation urinary hormone profiles and the probability of conception in healthy women. Fertil Steril. 1999;71:40–9.
Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet 2018;391:1842–52.
Blackburn ST. Maternal, fetal, and neonatal physiology. 3rd ed. St. Louis: Elsevier; 2007.
Lindheimer MD, Davison JM. Osmoregulation, the secretion of arginine vasopressin and its metabolism during pregnancy. Eur J Endocrinol. 1995;132:133–43.
Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54:2056–63.
de Alvarez RR, Bratvold GE. Renal glomerulotubular mechanisms during normal pregnancy. Am J Obstet Gynecol. 1958;75:931–44.
Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78:486–94.
Chadha V, Garg U, Alon US. Measurement of urinary concentration: a critical appraisal of methodologies. Pediatr Nephrol. 2001;16:374–82.
Weinberg CR, Umbach DM. Using pooled exposure assessment to improve efficiency in case-control studies. Biometrics 1999;55:718–26.
Saha-Chaudhuri P, Umbach DM, Weinberg CR. Pooled exposure assessment for matched case-control studies. Epidemiology 2011;22:704–12.
Saha-Chaudhuri P, Weinberg CR. Specimen pooling for efficient use of biospecimens in studies of time to a common event. Am J Epidemiol. 2013;178:126–35.
Acknowledgements
Many thanks to Dr D. Robert McConnaughey for his data management support, and to Drs Kaitlyn Gam and Kelly Ferguson for their comments on an earlier draft of this paper. This research was supported, in part, by the intramural research program of the NIH (project numbers ES103333-01 and ES103086), and by a doctoral fellowship at the Yale School of Public Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rosen Vollmar, A.K., Johnson, C.H., Weinberg, C.R. et al. Accounting for urinary dilution in peri-implantation samples: implications for creatinine adjustment and specimen pooling. J Expo Sci Environ Epidemiol 31, 356–365 (2021). https://doi.org/10.1038/s41370-020-0227-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41370-020-0227-1