Abstract
Background
Few studies have examined phthalate exposure during infancy and early life, critical windows of development. The Canadian Healthy Infant Longitudinal Development (CHILD) study, a population-based birth cohort, ascertained multiple exposures during early life.
Objective
To characterize exposure to phthalates during infancy and early childhood.
Methods
Environmental questionnaires were administered, and urine samples collected at 3, 12, and 36 months. In the first 1578 children, urine was analyzed for eight phthalate metabolites: mono-methyl phthalate (MMP), mono-ethyl phthalate (MEP), mono-butyl phthalate (MBP), mono-benzyl phthalate (MBzP), mono-2-ethylhexyl phthalate (MEHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and mono-3-carboxypropyl phthalate (MCPP). Geometric mean (GM) concentrations were calculated by age, together with factors that may influence concentrations. Trends with age were examined using mixed models and differences within factors examined using ANOVA.
Results
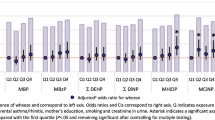
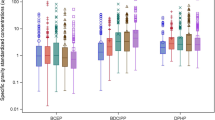
The highest urinary concentration was for the metabolite MBP at all ages (GM: 15–32 ng/mL). Concentrations of all phthalate metabolites significantly increased with age ranging from GM: 0.5–15.1 ng/mL at 3 months and 1.9–32.1 ng/mL at 36 months. Concentrations of all metabolites were higher in the lowest income categories except for MEHP at 3 months, among children with any breastfeeding at 12 months, and in urine collected on dates with warmer outdoor temperatures (>17 °C), except for MBzP at 3 months and MEHP at 3 and 12 months. No consistent differences were found by gender, study site, or maternal age.
Conclusions
Higher phthalate metabolite concentrations were observed among children in lower income families. Examination of factors associated with income could inform interventions aimed to reduce infant phthalate exposure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Health Canada. Results of the Canadian Health Measures Survey Cycle 2 (2009-2011), second report on human biomonitoring of environmental chemicals in Canada. Ottawa, Ontario: Health Canada; 2013. p. 1–434.
Lowell Center for Sustainable Production. Phthalates and their alternatives: health and environmental concerns. 2011. http://ec.europa.eu/environment/aarhus/pdf/35/Annex_11_report_from_Lowell_Center.pdf
Braun JM, Sathyanarayana S, Hauser R. Phthalate exposure and children’s health. Curr Opin Pediatr. 2013;25:247–54.
Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29:134–9.
Saravanabhavan G, Guay M, Langlois É, Giroux S, Murray J, Haines D. Biomonitoring of phthalate metabolites in the Canadian population through the Canadian Health Measures Survey (2007–2009). Int J Hyg Environ Health. 2013;216:652–61.
Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112:331–8.
Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol. 2003;37:4543–53.
Sathyanarayana S. Phthalates and children’s health. Curr Probl Pediatr Adolesc Health Care. 2008;38:34–49.
Kiyama R, Wada-Kiyama Y. Estrogenic endocrine disruptors: Molecular mechanisms of action. Environ Int. 2015;83:11–40.
Harris CA, Henttu P, Parker MG, Sumpter JP. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect. 1997;105:802–11.
Ghisari M, Bonefeld-Jorgensen EC. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol Lett. 2009;189:67–77.
Hashimoto Y, Moriguchi Y, Oshima H, Nishikawa J, Nishihara T, Nakamura M. Estrogenic activity of chemicals for dental and similar use in vitro. J Mater Sci Mater Med. 2000;11:465–8.
Ministry of Environment and Food of Denmark. EU ban harmful chemicals due to their “cocktail effects” [press release]. 2018. https://en.mfvm.dk/news/news/nyhed/eu-ban-harmful-chemicals-due-to-their-cocktail-effects/
Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010;118:565–71.
Cho S-C, Bhang S-Y, Hong Y-C, Shin M-S, Kim B-N, Kim J-W, et al. Relationship between environmental phthalate exposure and the intelligence of school-age children. Environ Health Perspect. 2010;118:1027–32.
Kim Y, Ha E-H, Kim E-J, Park H, Ha M, Kim J-H, et al. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children’s Environmental Health (MOCEH) study. Environ Health Perspect. 2011;119:1495–500.
Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–61.
Suzuki Y, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int J Androl. 2012;35:236–44.
Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999-2002. Environ Health. 2008;7:27.
Teitelbaum SL, Mervish N, Moshier EL, Vangeepuram N, Galvez MP, Calafat AM, et al. Associations between phthalate metabolite urinary concentrations and body size measures in New York City children. Environ Res. 2012;112:186–93.
International Agency for Research on Cancer (IARC). Monographs on the evaluation of carcinogenic risks to human, volume 101: some chemicals present in industrial and consumer products, food and drinking-water. Lyon, France: IARC; 2013.
Bornehag C-G, Sundell J, Weschler CJ, Sigsgaard T, Lundgren B, Hasselgren M, et al. The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environ Health Perspect. 2004;112:1393–7.
Whyatt RM, Perzanowski MS, Just AC, Rundle AG, Donohue KM, Calafat AM, et al. Asthma in inner-city children at 5-11 years of age and prenatal exposure to phthalates: the Columbia Center for Children’s Environmental Health Cohort. Environ Health Perspect. 2014;122:1141–6.
Ku HY, Su PH, Wen HJ, Sun HL, Wang CJ, Chen HY, et al. Prenatal and postnatal exposure to phthalate esters and asthma: a 9-year follow-up study of a taiwanese birth cohort. PloS One. 2015;10:1–14.
Gascon M, Casas M, Morales E, Valvi D, Ballesteros-Gómez A, Luque N, et al. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J Allergy Clin Immunol. 2015;135:370–8.
Shu H, Jönsson BA, Larsson M, Nånberg E, Bornehag C-G. PVC flooring at home and development of asthma among young children in Sweden, a 10-year follow-up. Indoor Air. 2014;24:227–35.
Callesen M, Bekö G, Weschler CJ, Langer S, Brive L, Clausen G, et al. Phthalate metabolites in urine and asthma, allergic rhinoconjunctivitis and atopic dermatitis in preschool children. Int J Hyg Environ Health. 2014;217:645–52.
Ait Bamai Y, Shibata E, Saito I, Araki A, Kanazawa A, Morimoto K, et al. Exposure to house dust phthalates in relation to asthma and allergies in both children and adults. Sci Total Environ. 2014;485–486:153–63.
Hoppin JA, Jaramillo R, London SJ, Bertelsen RJ, Salo PM, Sandler DP, et al. Phthalate exposure and allergy in the U.S. population: results from NHANES 2005-2006. Environ Health Perspect. 2013;121:1129–34.
Bornehag CG, Nanberg E. Phthalate exposure and asthma in children. Int J Androl. 2010;33:333–45.
Johns LE, Cooper GS, Galizia A, Meeker JD. Exposure assessment issues in epidemiology studies of phthalates. Environ Int. 2015;85:27–39.
Wittassek M, Angerer J. Phthalates: metabolism and exposure. Int J Androl. 2008;31:131–8.
Frederiksen H, Kranich SK, Jørgensen N, Taboureau O, Petersen JH, Andersson A-M. Temporal variability in urinary phthalate metabolite excretion based on spot, morning, and 24-h urine samples: considerations for epidemiological studies. Environ Sci Technol. 2013;47:958–67.
Barker DJP. Sir Richard doll lecture. Developmental origins of chronic disease. Public Health. 2012;126:185–9.
Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2017;13:161–73.
Kim S, Lee J, Park J, Kim H-J, Cho GJ, Kim G-H, et al. Urinary phthalate metabolites over the first 15months of life and risk assessment—CHECK cohort study. Sci Total Environ. 2017;607–608:881–7.
Arbuckle TE, Fisher M, MacPherson S, Lang C, Provencher G, LeBlanc A, et al. Maternal and early life exposure to phthalates: the plastics and personal-care products use in pregnancy (P4) study. Sci Total Environ. 2016;551–552:344–56.
Frederiksen H, Kuiri-Hänninen T, Main KM, Dunkel L, Sankilampi U. A longitudinal study of urinary phthalate excretion in 58 full-term and 67 preterm infants from birth through 14 months. Environ Health Perspect. 2014;122:998–1005.
Völkel W, Kiranoglu M, Schuster R, Fromme H, HBMnet. Phthalate intake by infants calculated from biomonitoring data. Toxicol Lett. 2014;225:222–9.
Sathyanarayana S, Karr CJ, Lozano P, Brown E, Calafat AM, Liu F, et al. Baby care products: possible sources of infant phthalate exposure. Pediatrics. 2008;121:e260–8.
Subbarao P, Anand SS, Becker AB, Befus AD, Brauer M, Brook JR, et al. The Canadian Healthy Infant Longitudinal Development (CHILD) study: examining developmental origins of allergy and asthma. Thorax. 2015;70:998–1000.
Takaro TK, Scott JA, Allen RW, Anand SS, Becker AB, Befus AD, et al. The Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort study: assessment of environmental exposures. J Expo Sci Environ. Epidemiol 2015;25:580–92.
Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem 2005;77:2985–91.
Helsel DR. Nondetects and data analysis. Statistics for censored environmental data. Hoboken, USA: Wiley-Interscience; 2005. p. 250 https://www.cabdirect.org/cabdirect/abstract/20053102639.
Carrieri M, Trevisan A, Bartolucci GB. Adjustment to concentration-dilution of spot urine samples: correlation between specific gravity and creatinine. Int Arch Occup Environ Health. 2001;74:63–7.
Albro PW, Lavenhar SR. Metabolism of di(2-ethylhexyl)phthalate. Drug Metab Rev. 1989;21:13–34.
Barr DB, Silva MJ, Kato K, Reidy JA, Malek NA, Hurtz D, et al. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ Health Perspect. 2003;111:1148–51.
Albro PW, Corbett JT, Schroeder JL, Jordan S, Matthews HB. Pharmacokinetics, interactions with macromolecules and species differences in metabolism of DEHP. Environ Health Perspect. 1982;45:19–25.
Schmid P, Schlatter C. Excretion and metabolism of di(2-ethylhexyl)phthalate in man. Xenobiotica. 1985;15:251–6.
Dirven HA, van den Broek PH, Jongeneelen FJ. Determination of four metabolites of the plasticizer di(2-ethylhexyl)phthalate in human urine samples. Int Arch Occup Environ Health. 1993;64:555–60.
Becker K, Seiwert M, Angerer J, Heger W, Koch HM, Nagorka R, et al. DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health. 2004;207:409–17.
Firestone M, Moya J, Cohen‐Hubal E, Zartarian V, Xue J. Identifying childhood age groups for exposure assessments and monitoring. Risk Anal. 2007;27:701–14.
Carlstedt F, Jönsson BaG, Bornehag C-G. PVC flooring is related to human uptake of phthalates in infants. Indoor Air. 2013;23:32–9.
Lin S, Ku H-Y, Su P-H, Chen J-W, Huang P-C, Angerer J, et al. Phthalate exposure in pregnant women and their children in central Taiwan. Chemosphere. 2011;82:947–55.
Becker K, Goen T, Seiwert M, Conrad A, Pick-Fuss H, Muller J, et al. GerES IV: phthalate metabolites and bisphenol A in urine of German children. Int J Hyg Environ Health. 2009;212:685–92.
Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001-2010. Environ Health Perspect. 2014;122:235–41.
Statistics Canada. Table 11-10-0011-01 Census families by age of older partner or parent and number of children. Statista. https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1110001101#timeframe
Wenzel AG, Brock JW, Cruze L, Newman RB, Unal ER, Wolf BJ, et al. Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere. 2018;193:394–402.
Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environ Res. 2008;108:260–7.
Jung K, Oh H, Ryu JY, Kim DH, Lee S, Son B-C, et al. Relationship between housing characteristics, lifestyle factors and phthalates exposure: the first Korean National Environmental Health Survey (2009–2011). Ann Occup Environ Med. 2015;27:33.
Casas L, Fernández MF, Llop S, Guxens M, Ballester F, Olea N, et al. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int. 2011;37:858–66.
Kobrosly RW, Parlett LE, Stahlhut RW, Barrett ES, Swan SH. Socioeconomic factors and phthalate metabolite concentrations among United States women of reproductive age. Environ Res. 2012;115:11–7.
Tyrrell J, Melzer D, Henley W, Galloway TS, Osborne NJ. Associations between socioeconomic status and environmental toxicant concentrations in adults in the USA: NHANES 2001-2010. Environ Int. 2013;59:328–35.
Shea KM. Pediatric exposure and potential toxicity of phthalate plasticizers. Pediatrics. 2003;111:1467–74.
Zota AR, Phillips CA, Mitro SD. Recent Fast Food Consumption and Bisphenol A and Phthalates Exposures among the U.S. Population in NHANES, 2003-2010. Environ Health Perspect. 2016;124:1521–8.
French SA, Wall M, Mitchell NR. Household income differences in food sources and food items purchased. Int J Behav Nutr Phys Act. 2010;7:77.
Power EM. Determinants of healthy eating among low-income Canadians. Can J Public Health. 2005;96:S37–42.
Drewnowski A, Specter SE. Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr. 2004;79:6–16.
Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health. 2014;13:43.
Kashyap D, Agarwal T. Concentration and factors affecting the distribution of phthalates in the air and dust: A global scenario. Sci Total Environ. 2018;635:817–27.
Ait Bamai Y, Araki A, Kawai T, Tsuboi T, Saito I, Yoshioka E, et al. Associations of phthalate concentrations in floor dust and multi-surface dust with the interior materials in Japanese dwellings. Sci Total Environ. 2014;468–469:147–57.
Liang Y, Xu Y. Emission of phthalates and phthalate alternatives from vinyl flooring and crib mattress covers: the influence of temperature. Environ Sci Technol. 2014;48:14228–37.
Bi C, Liang Y, Xu Y. Fate and transport of phthalates in indoor environments and the influence of temperature: a case study in a test house. Environ Sci Technol. 2015;49:9674–81.
Wei W, Mandin C, Ramalho O. Influence of indoor environmental factors on mass transfer parameters and concentrations of semi-volatile organic compounds. Chemosphere. 2018;195:223–35.
Clausen PA, Liu Z, Kofoed-Sørensen V, Little J, Wolkoff P. Influence of temperature on the emission of di-(2-ethylhexyl)phthalate (DEHP) from PVC flooring in the emission cell FLEC. Environ Sci Technol. 2012;46:909–15.
Donahue NM, Robinson AL, Stanier CO, Pandis SN. Coupled partitioning, dilution, and chemical aging of semivolatile organics. Environ Sci Technol. 2006;40:2635–43.
Gao H, Zhu Y-D, Xu Y-Y, Zhang Y-W, Yao H-Y, Sheng J, et al. Season-dependent concentrations of urinary phthalate metabolites among Chinese pregnant women: repeated measures analysis. Environ Int. 2017;104:110–7.
Shu H, Jönsson BA, Gennings C, Svensson Å, Nånberg E, Lindh CH, et al. Temporal trends of phthalate exposures during 2007-2010 in Swedish pregnant women. J Expo Sci Environ Epidemiol. 2018;28:437–47.
Gilbert ES. The impact of dosimetry uncertainties on dose-response analyses. Health Phys. 2009;97:487–92.
Whitehead TP, Nuckols JR, Ward MH, Rappaport SM. Carpet-dust chemicals as measures of exposure: Implications of variability. Emerg Themes Epidemiol. 2012;9:2.
Bennett DH, Moran RE, Wu XM, Tulve NS, Clifton MS, Colón M, et al. Polybrominated diphenyl ether (PBDE) concentrations and resulting exposure in homes in California: relationships among passive air, surface wipe and dust concentrations, and temporal variability. Indoor Air. 2015;25:220–9.
Acknowledgements
We are grateful to the CHILD study families for their participation and the entire CHILD study team including interviewers, research assistants, nurses, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers and receptionists. We also thank Jerome Lavoue for his support with NDExpo. Funding for this study was provided through the Canadian Institutes of Health Research (CIHR), Allergy, Genes and Environment (AllerGen) Network of Centers of Excellence and the Government of Canada’s Chemical Management Plan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Navaranjan, G., Takaro, T.K., Wheeler, A.J. et al. Early life exposure to phthalates in the Canadian Healthy Infant Longitudinal Development (CHILD) study: a multi-city birth cohort. J Expo Sci Environ Epidemiol 30, 70–85 (2020). https://doi.org/10.1038/s41370-019-0182-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41370-019-0182-x
Keywords
This article is cited by
-
High exposure to phthalates is associated with HbA1c worsening in type 2 diabetes subjects with and without edentulism: a prospective pilot study
Diabetology & Metabolic Syndrome (2022)
-
Indoor exposure to phthalates and polycyclic aromatic hydrocarbons (PAHs) to Canadian children: the Kingston allergy birth cohort
Journal of Exposure Science & Environmental Epidemiology (2022)