Abstract
Background
The general population is exposed to phthalates, a group of chemicals with strong evidence for endocrine disrupting properties, commonly used in a large number of consumer products. Based on published research and evidence compiled by environmental agencies, certain phthalate applications and products have become restricted, leading to an increasing number of “new generation compounds” coming onto the market during recent years replacing older phthalates. Some examples of such newer compounds are di-iso-nonyl phthalate (DiNP), di-iso-decyl phthalate (DiDP), and most recently di-isononyl-cyclohexane-1,2-dicarboxylate (DiNCH).
Objectives
In order to evaluate temporal trends in phthalate exposure, first trimester urinary biomarkers of phthalates were measured in the Swedish SELMA study over a period of 2.5 years (2007–2010).
Methods
We collected first morning void urine samples around week 10 of pregnancy from 1651 pregnant women. Spot samples were analyzed for 13 phthalate metabolites and one phthalate replacement and least square geometric mean (LSGM) levels of the metabolites were compared between the sampling years when adjusted for potential confounders.
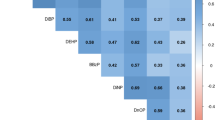
Results
All 14 metabolites were detectable in more than 99% of the SELMA subjects. The levels were generally comparable to other studies, but the SELMA subjects showed slightly higher exposure to butyl-benzyl phthalate (BBzP) and di-butyl phthalate (DBP). Di-ethyl-hexyl phthalate (DEHP) metabolites levels decreased while DiNP, DiDP/di-2-propylheptyl phthalate (DPHP), and DiNCH metabolites levels increased during the sampling period.
Conclusions
Urinary metabolite levels of the older phthalates and more recently introduced phthalate replacement compound changed during the short sampling period in this Swedish pregnancy cohort. Our results indicate that replacement of phthalates can make an impact on human exposure to these chemicals. During this particularly vulnerable stage of life, phthalate exposures are of particular concern as the impacts, though not immediately noticeable, may increase the risk for health effects later in life.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bergman A, Heindel JJ, Jobling MS, Kidd KA, Zoeller RT. State of the science of endocrine disrupting chemicals 2012: an assessment of the state of the science of endocrine disruptors prepared by a group of experts for the United Nations Environment Programme and World Health Organization. World Health Organization; 2013. www.who.int/iris/bitstream/10665/78101/1/9789241505031_eng.pdf
Carlstedt F, Jonsson BA, Bornehag CG. PVC flooring is related to human uptake of phthalates in infants. Indoor Air. 2013;23:32–9.
Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119:914–20.
Sathyanarayana S, Karr CJ, Lozano P, Brown E, Calafat AM, Liu F, et al. Baby care products: possible sources of infant phthalate exposure. Pediatrics. 2008;121:e260–8.
Wittassek M, Koch HM, Angerer J, Bruning T. Assessing exposure to phthalates—the human biomonitoring approach. Mol Nutr Food Res. 2011;55:7–31.
Bergh C, Torgrip R, Emenius G, Ostman C. Organophosphate and phthalate esters in air and settled dust—a multi-location indoor study. Indoor Air. 2011;21:67–76.
Rudel RA, Perovich LJ. Endocrine disrupting chemicals in indoor and outdoor air. Atmos Environ. 2009;43:170–81.
Bornehag CG, Lundgren B, Weschler CJ, Sigsgaard T, Hagerhed-Engman L, Sundell J. Phthalates in indoor dust and their association with building characteristics. Environ Health Perspect. 2005;113:1399.
Bornehag CG, Sundell J, Weschler CJ, Sigsgaard T, Lundgren B, Hasselgren M, et al. The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environ Health Perspect. 2004;112:1393–7.
Qi Z, Xiao-Mei L, Xiao-Ling Z, Yong-Gang S, Dong-Mei Z, Bing-Ling W, et al. Levels of phthalate esters in settled house dust from urban dwellings with young children in Nanjing, China. Atmos Environ. 2013;69:258–64.
Langer S, Beko G, Weschler CJ, Brive LM, Toftum J, Callesen M, et al. Phthalate metabolites in urine samples from Danish children and correlations with phthalates in dust samples from their homes and daycare centers. Int J Hyg Environ Health. 2013;217:78–87.
Shi W, Hu X, Zhang F, Hu G, Hao Y, Zhang X, et al. Occurrence of thyroid hormone activities in drinking water from eastern China: contributions of phthalate esters. Environ Sci Technol. 2012;46:1811–8.
Cirillo T, Fasano E, Castaldi E, Montuori P, Amodio Cocchieri R. Children’s exposure to Di(2-ethylhexyl)phthalate and dibutylphthalate plasticizers from school meals. J Agric Food Chem. 2011;59:10532–8.
Fierens T, Servaes K, Van Holderbeke M, Geerts L, De Henauw S, Sioen I, et al. Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem Toxicol: Int J Publ Br Ind Biol Res Assoc. 2012;50:2575–83.
Dhanya CR, Gayathri NS, Mithra K, Nair KV, Kurup PA. Vitamin E prevents deleterious effects of di (2-ethyl hexyl) phthalate, a plasticizer used in PVC blood storage bags. Indian J Exp Biol. 2004;42:871–5.
Hauser R, Duty S, Godfrey-Bailey L, Calafat AM. Medications as a source of human exposure to phthalates. Environ Health Perspect. 2004;112:751–3.
Bajkin I, Bjelica A, Icin T, Dobric V, Zavisic BK, Stojanoska MM. Effects of phthalic acid esters on fetal health. Med Pregl. 2014;67:172–5.
Lopez-Carrillo L, Hernandez-Ramirez RU, Calafat AM, Torres-Sanchez L, Galvan-Portillo M, Needham LL, et al. Exposure to phthalates and breast cancer risk in northern Mexico. Environ Health Perspect. 2010;118:539–44.
Cho SC, Bhang SY, Hong YC, Shin MS, Kim BN, Kim JW, et al. Relationship between environmental phthalate exposure and the intelligence of school-age children. Environ Health Perspect. 2010;118:1027–32.
Kim BN, Cho SC, Kim Y, Shin MS, Yoo HJ, Kim JW, et al. Phthalates exposure and attention-deficit/hyperactivity disorder in school-age children. Biol Psychiatry. 2009;66:958–63.
Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010;118:565–71.
Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–61.
Jurewicz J, Hanke W. Exposure to phthalates: reproductive outcome and children health. A review of epidemiological studies. Int J Occup Med Environ Health. 2011;24:115–41.
Bornehag CG, Nanberg E. Phthalate exposure and asthma in children. Int J Androl. 2010;33:333–45.
Edna R, Carina L, Susana V. EDCs mixtures: a stealthy hazard for human health? Toxics. 2017;5(Iss 1):5. p2017(1):5
Johnson S, Saikia N, Sahu R. Phthalates in toys available in Indian market. Bull Environ Contam Toxicol. 2011;86:621–6.
Cate MT. The birth of plastic. ICIS Chem Bus. 2009;275:26–7.
European Union. Commission delegated directive (EU) 2015/863, amending Annex II to Directive 2011/65/EU of the European Parliament and of the Council as regards the list of restricted substances 2015. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32015L0863.
KEMI SCA. Phthalates which are toxic for reproduction and endocrine-disrupting—proposals for a phase-out in Sweden. Stockholm: Arkitektkopia; 2015.
Van Vliet ED, Reitano EM, Chhabra JS, Bergen GP, Whyatt RM. A review of alternatives to di (2-ethylhexyl) phthalate-containing medical devices in the neonatal intensive care unit. J Perinatol. 2011;31:551–60.
Koch HM, Schutze A, Palmke C, Angerer J, Bruning T. Metabolism of the plasticizer and phthalate substitute diisononyl-cyclohexane-1,2-dicarboxylate (DINCH((R))) in humans after single oral doses. Arch Toxicol. 2012;87:799–806.
Bornehag CG, Moniruzzaman S, Larsson M, Lindstrom CB, Hasselgren M, Bodin A, et al. The SELMA study: a birth cohort study in Sweden following more than 2000 mother-child pairs. Paediatr Perinat Epidemiol. 2012;26:456–67.
Bornehag CG, Carlstedt F, Jonsson BA, Lindh CH, Jensen TK, Bodin A, et al. Prenatal phthalate exposures and anogenital distance in Swedish boys. Environ Health Perspect. 2015;123:101–7.
Gyllenhammar I, Glynn A, Jonsson BA, Lindh CH, Darnerud PO, Svensson K, et al. Diverging temporal trends of human exposure to bisphenols and plastizisers, such as phthalates, caused by substitution of legacy EDCs? Environ Res. 2017;153:48–54.
Mazzachi BC, Peake MJ, Ehrhardt V. Reference range and method comparison studies for enzymatic and Jaffe creatinine assays in plasma and serum and early morning urine. Clin Lab. 2000;46:53–5.
Gries W, Ellrich D, Kupper K, Ladermann B, Leng G. Analytical method for the sensitive determination of major di-(2-propylheptyl)-phthalate metabolites in human urine. J Chromatogr B Anal Technol Biomed Life Sci. 2012;908:128–36.
Lindh CH, Rylander L, Toft G, Axmon A, Rignell-Hydbom A, Giwercman A, et al. Blood serum concentrations of perfluorinated compounds in men from Greenlandic Inuit and European populations. Chemosphere. 2012;88:1269–75.
Jefferis BJ, Lawlor DA, Ebrahim S, Wannamethee SG, Feyerabend C, Doig M, et al. Cotinine-assessed second-hand smoke exposure and risk of cardiovascular disease in older adults. Heart. 2010;96:854.
Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect. 2014;122:235–41.
Schutze A, Kolossa-Gehring M, Apel P, Bruning T, Koch HM. Entering markets and bodies: increasing levels of the novel plasticizer Hexamoll(R) DINCH(R) in 24 h urine samples from the German Environmental Specimen Bank. Int J Hyg Environ Health. 2014;217:421–6.
KEMI SCA PVC—Turnover of the biggest thermoplastics 2011. http://www3.kemi.se/en/Content/Statistics/Statistics-in-brief/Statistics-in-brief---Substances-and-substance-groups/PVC/.
Shu H, Jonsson BA, Larsson M, Nanberg E, Bornehag CG. PVC flooring at home and development of asthma among young children in Sweden, a 10-year follow-up. Indoor Air. 2014;24:227–35.
Zota AR, Phillips CA, Mitro SD. Recent fast food consumption and bisphenol A and phthalates exposures among the U.S. population in NHANES, 2003-2010. Environ Health Perspect. 2016;124:1521–8.
Arbuckle TE, Fisher M, MacPherson S, Lang C, Provencher G, LeBlanc A, et al. Maternal and early life exposure to phthalates: the plastics and personal-care products use in pregnancy (P4) study. Sci Total Environ. 2016;551-552:344–56.
Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, et al. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect. 2012;120:464–70.
Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environ Res. 2008;108:260–7.
Casas L, Fernandez MF, Llop S, Guxens M, Ballester F, Olea N, et al. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int. 2011;37:858–66.
Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, et al. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environ Int. 2014;62:1–11.
Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008;116:467–73.
Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RH, et al. First trimester phthalate exposure and anogenital distance in newborns. Human Reprod. 2015;30:963–72.
Koch HM, Rüther M, Schütze A, Conrad A, Pälmke C, Apel P, et al. Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int J Hyg Environ Health. 2017;220(2, Part A):130–41.
Saravanabhavan G, Guay M, Langlois E, Giroux S, Murray J, Haines D. Biomonitoring of phthalate metabolites in the Canadian population through the Canadian Health Measures Survey (2007-2009). Int J Hyg Environ Health. 2013;216:652–61.
Tellez-Rojo MM, Cantoral A, Cantonwine DE, Schnaas L, Peterson K, Hu H, et al. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at 2 and 3 years of age. Sci Total Environ. 2013;461–462:386–90.
Calafat AM, Koch HM, Swan SH, Hauser R, Goldman LR, Lanphear BP, et al. Misuse of blood serum to assess exposure to bisphenol A and phthalates. Breast Cancer Res. 2013;15:403.
Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect. 2002;110:515–8.
Acknowledgements
The study was funded by grants from the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas), Swedish Asthma and Allergy Association’s Research Foundation, the Swedish Foundation for Health Care Sciences and Allergy Research and the County Council of Värmland. We also thank all the participating families.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shu, H., Jönsson, B.A., Gennings, C. et al. Temporal trends of phthalate exposures during 2007–2010 in Swedish pregnant women. J Expo Sci Environ Epidemiol 28, 437–447 (2018). https://doi.org/10.1038/s41370-018-0020-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41370-018-0020-6
Keywords
This article is cited by
-
Prenatal exposure to common plasticizers: a longitudinal study on phthalates, brain volumetric measures, and IQ in youth
Molecular Psychiatry (2023)
-
Prenatal phthalate exposure and early childhood wheeze in the SELMA study
Journal of Exposure Science & Environmental Epidemiology (2022)
-
Phthalate levels in indoor dust and associations to croup in the SELMA study
Journal of Exposure Science & Environmental Epidemiology (2021)
-
Prenatal exposures to mixtures of endocrine disrupting chemicals and children’s weight trajectory up to age 5.5 in the SELMA study
Scientific Reports (2021)
-
Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico
Journal of Exposure Science & Environmental Epidemiology (2020)