Abstract
Considering the substantial role played by dendritic cells (DCs) in the immune system to bridge innate and adaptive immunity, studies on DC-mediated immunity toward biomaterials principally center on their adjuvant effects in facilitating the adaptive immunity of codelivered antigens. However, the effect of the intrinsic properties of biomaterials on dendritic cells has not been clarified. Recently, researchers have begun to investigate and found that biomaterials that are nonadjuvant could also regulate the immune function of DCs and thus affect subsequent tissue regeneration. In the case of proteins adsorbed onto biomaterial surfaces, their intrinsic properties can direct their orientation and conformation, forming “biomaterial-associated molecular patterns (BAMPs)”. Thus, in this review, we focused on the intrinsic physiochemical properties of biomaterials in the absence of antigens that affect DC immune function and summarized the underlying signaling pathways. Moreover, we preliminarily clarified the specific composition of BAMPs and the interplay between some key molecules and DCs, such as heat shock proteins (HSPs) and high mobility group box 1 (HMGB1). This review provides a new direction for future biomaterial design, through which modulation of host immune responses is applicable to tissue engineering and immunotherapy.
Similar content being viewed by others
Introduction
Biomaterials and biomedical devices implanted in a host trigger a series of orchestrated immune reactions, including blood-biomaterial contact, the formation of adsorbed proteins, and recruitment of immune cells (Fig. 1a, b).1 Successful integration of implanted biomaterials requires complete removal of inflammation and tissue regeneration, which is highly related to complex immunity. In this context, ongoing efforts have been devoted to excavating the interplay of biomaterials and host immune systems, the involved signaling molecules, and the underlying mechanisms.2 Biologically distinct subsets of DCs originate from hematopoietic stem cells (HSCs) in the bone marrow and regulate the function of T cells in different ways,3 including myeloid/conventional DCs, plasmatic DCs, and follicular DCs.4,5,6 As the most efficient antigen-presenting cells (APCs), they serve as a jointing band between innate and adaptive immunity and maintain a core position in the process of injury and healing. Recent knockout studies have identified the critical role of DCs in immune responses.7 For instance, conditional and constitutive ablation of DCs in mice might lead to spontaneous development of autoimmune disease.8
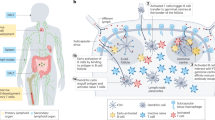
Important immune events involved in host response towards implanted biomaterials. a Host proteins majorly adhere to the surface of biomaterials within 4 h, which are followed by a series of subsequential chain responses like the infiltration of immune cells consecutively (see Supplementary Fig. S1 for detailed illustration). b DCs surveying in the periphery signal foreign biomaterials through BAMP. Meanwhile, driven by cytokines and chemokines released from the surrounding lymphocytes, DCs undergo the transformation towards mDCs or tolDCs. c Specifically, having recognized the biomaterial through BAMP, iDCs experience the shift towards antigen-capturing mDCs with a ‘stellate’ morphology and upregulated expression of stimulatory and co-stimulatory molecules such as MHC-II, CD80, and CD86, which further initiated T cell-mediated adaptive immune response. On the contrary, iDCs encountering self-antigens or in the absence of foreign signals would differentiate into tolDCs, which downregulate the expression of co-stimulatory molecules, enhance the expression of inhibitory molecules, and promote anti-inflammatory cytokines TGF-β and IL-10. *The arrows with solid lines in two different colors both indicate a progressive meaning, with the red arrow involved in inflammation and the blue one in immunomodulation. In addition, the double-ended arrow in the right part of Fig. 1(c) indicates that productions from T cell could also induce tolDCs differentiation (e.g., TGF-β, IL-10)
Under steady state, DCs surveying in the periphery signal foreign antigens through pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), thus generating a battery of immune reactions.9,10 During the process, pattern-recognizing receptors (PRRs) are adopted by immature tissue-resident DCs (iDCs) to capture antigens, including Toll-like receptors (TLRs), C-type lectin receptors (CLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs).10 Initiated by foreign signals, iDCs are transferred into mature DCs (mDCs), which are capable of launching T-cell mediated immune responses against foreign attacks.11 iDCs encountering self-antigens or in the absence of foreign signals differentiate into tolerogenic DCs (tolDCs), which promote immune tolerance.12 The double-edged role of DCs seems to have been extensively studied for orchestrating immune responses,1 of which plasticity is generated from their unique role as sentinels, consecutively signaling their peripheral cues and exerting context-dependent properties.13,14 Considering this, recent studies have revealed that modifying the physiochemical properties of implanted biomaterials would generate exceptionally different immune milieus and elicit varied DC-mediated host responses,15 thus leading to immune responses or tolerance.16,17,18 For instance, genetically modified19,20,21 or pharmacologically conditioned22,23,24 maturation-resistant DCs have been introduced to promote allograft survival, tissue regeneration, vaccine delivery,25 autoimmune disease treatment26, and tumor immunotherapies. Therefore, a comprehensive elucidation of the interplay between biomaterial physiochemical properties and DC phenotypes is truly important.27 Based on this, future efforts might be attributed to inducing tolerogenic, ‘alternatively activated’, and effective mDCs via biomaterial surface modifications in an attempt to disseminate the profound application of biomaterials.28,29 Nevertheless, within the limit of our knowledge, approaches to modulate the function of DCs have historically focused on inducing the bioinertness of the materials but overlook their intrinsic bioactivity, which is critical for tissue regeneration.30 Moreover, the underlying mechanisms involved in DC-biomaterial interactions have not been well established yet. In particular, the definition and connotation of BAMP has been hardly expounded upon, much less the idiographic chemical composition and structures. Herein, we provide an in-depth look at the current knowledge on the multifaceted effects of the physiochemical properties of biomaterials on DC biofunction and explicate DC behaviors toward implanted biomaterials, with a specific emphasis on the underlying mechanisms. We believe that understanding this complex scenario will assist in the innovative design and clinical application of biomaterials with desired immune effects in tumor therapy, vaccine delivery, and tissue engineering.
Host response to implanted biomaterials
Biomedical devices or materials implanted in a host universally trigger a highly orchestrated chain of events, including inflammation, and the balance of the immune system breaks down when inflammation is excessive or becomes chronic.31,32 When implanted into the host, biomaterial comes into contact with blood or humoral fluids, and proteins will instantly adsorb to the surface of the material.1 Generally, host proteins primarily adhere to the surface within 4 h, which is followed by a series of subsequent chain responses occurring on the surface of the biomaterial, including complement activation, the coagulation cascade, and immune cell adhesion.1 Early infiltration of the neutrophil population was observed in minutes to hours according to Leifer,32 which lasted until 24–48 h after implantation and was followed by monocyte accumulation on biomaterials as well as differentiation into macrophages by 72 h postimplantation.33,34,35
With a relatively limited proportion of approximately 0.5%–1.5% of the total mononuclear cells in peripheral blood, as well as short lifetimes of days to weeks,34,36,37 DCs serve as the center of the immune system, providing a vital link between innate and adaptive immune responses.10,38,39 In contact with biomaterials, iDCs experience a shift toward antigen-capturing mDCs (Fig. 1c), which are characterized by a ‘stellate’ morphology composed of actin.40 Apart from this, many coordinated events are involved in DC maturation, including the upregulated expression of peptide-major histocompatibility (MHC)-I and -II complexes permitting effective antigen presentation, enhanced expression of adhesion molecules and chemokine receptors, and prominent allo-stimulatory capacity and pro-inflammatory cytokine secretion profiles, including IL-1β, IL-6, and IL-12p70.10,41,42,43 As evidenced by Yoshida, markedly increased presentation of costimulatory molecules, including CD80, CD83, and CD86, was observed at early time points (6 h, 24 h) on DCs treated with 75:25 poly(lactic-co-glycolic acid) (PLGA) films and MPs.44 As a result of the development of a repertoire of chemokine receptors, including CCR7,45,46 mDCs spontaneously migrate to local lymph nodes.11 Subsequently, T-helper cells were spotted as early as the third day locally at the implantation sites and in the contralateral bone marrow according to a mouse model, signaling the arousal of adaptive immune responses, which lasted over seven days.47 DC-mediated T-cell activation depends on three major signals: (i) the first signal refers to the combination of MHC-peptide complexes with antigen-specific TCRs; (ii) recognition and involvement of costimulatory molecules exhibited on DCs, including CD80 and CD86, by their receptors on the surface of T cells (e.g., CD28, CD40, CTLA-4), which constitutes the second signal; (iii) other soluble or membrane-bound signals, such as IL-12 and type I interferon (IFN-I), consisting of a “polarizing” signal.48
In contrast, iDCs encountering certain biomaterial signals, which have not been fully elaborated, differentiate into tolDCs.12 TolDCs preserve the ability of antigen presentation to antigen-specific T cells while downregulating the expression of costimulatory molecules (e.g., CD80, CD86, CD40) or proinflammatory cytokines, including IL-12, and enhancing the expression of coinhibitory molecules (e.g., IgG-like transcript 3, PDL1) and anti-inflammatory cytokines, such as transforming growth factor-β (TGF-β) and interleukin-10 (IL-10)49,50,51,52,53,54 (Fig. 1c). Immunological tolerance was maintained by inducing T-cell apoptosis, unresponsive T-cell responses, Treg cell production and inhibition of T-cell responses.8,9,27,55,56,57
Biomaterials properties modulating DC response
Modulating the immune-inflammatory axis via biomaterials can significantly ameliorate the efficacy of tissue engineering.58 Recently, an increasing number of studies have incorporated biomedical polymers with immunogenic or immunomodulatory molecules in an effort to regulate host responses to implanted biomaterials. Nevertheless, the influence of the intrinsic physiochemical properties of implanted biomaterials on DC phenotype and function has been overlooked. A better understanding of this complex interplay will help to modify the particular immune function while eliminating unexpected immune reactions.59 To date, many studies have focused on specific material properties, including chemical composition, surface chemistry, hydrophilicity, topography and roughness, spatial structures, surface charge, and incorporated bioactive inorganic ions, which are comprehensively characterized and discussed separately in this section and are summarized in Table S1 and Fig. 2.
Biomaterials properties modulating DC response. Effects of physiochemical properties of biomaterials that potentially influence DC phenotype and function are summarized in five columns: surface chemistry, hydrophilicity, topography and spatial structures, roughness, and surface charge. Conjugated peptides, chemical moieties, and elemental concentration can surely determine DCs behavior, and DCs seeded on agarose, chitosan or PLGA would boost DC maturation, whereas those treated with alginate or HA were associated with immune-tolerance. Furthermore, PLA with higher hydrophobicity than PELA could promoted effective antigen uptake and induced a higher level of DC maturation. However, DCs seeded on 3D scaffolds can either mature increasingly (e.g., collagen-chitosan scaffolds, pHEMA, and PDMS) or decreasingly (e.g., 3D collagen microenvironment) in comparison with those cultured on 2D culture plates. DCs seeded on titanium substrates with similar surface roughness (SLA, modSLA) presented different maturation. DOTAP/DOPC liposome, with a relatively cationic charge, potently promoted DC maturation, while negatively charged GQD could significantly impair DC-mediated immune reactions. Notably, the paired arrows with two directions in the figure indicated that contrasting outcomes were reported in terms of their effects on DC activation, and no definitive judgment could thus far be drawn. Specifically, these material properties may interact synergistically with each other to yield a microenvironment harmful to or favorable for tissue repair and regeneration. *Abbreviations: PLA poly(D,L-lactic acid), PELA poly(monomethoxypolyethylene glycol-co-D,L-lactide), pHEMA poly(2-hydroxyethyl methacrylate), PDMS poly(dimethylsiloxane), GQD graphene quantum dot
Surface chemistrypatibility and tissue regeneration
Biomaterial-induced immune responses are directly determined by the surface chemistry, such as conjugated peptides or chemical moieties, as well as elemental concentrations of carbon, oxygen, and nitrogen.60 Organic biomaterials, including agarose, alginate, chitosan, hyaluronic acid (HA), and 75:25 PLGA, are commonly applied in tissue engineering61 and display immunologic roles in the microenvironment.62 Yoshida et al. explored the effects of agarose and 75:25 PLGA MPs and films on human monocyte-derived DCs63 and murine bone marrow-derived DCs.44 They reported that under treatment with PLGA and agarose MPs or films, DCs, regardless of cell origin, showed an upregulation in expression of MHC II and costimulatory molecules, such as CD80 and CD86, in contrast to iDCs but to a lesser extent than that of lipopolysaccharide (LPS)-induced DCs. Babensee generated biofilms of five materials (agarose, alginate, chitosan, HA, PLGA) by casting techniques and examined their influences on iDCs derived from human peripheral blood monocytes.62 They found that chitosan and PLGA (and agarose to a lower level) films could promote DC costimulatory capacity equivalent to LPS-induced DCs, whereas alginate or HA films inhibited the costimulatory expression of DCs. Similarly, Park et al. found that cultivating DCs with alginate and HA did not stimulate DCs, while DCs seeded on PLGA or chitosan films presented upregulated surface maturation molecules, including CD80, CD86, CD83, and human leukocyte antigen-DQ (HLA-DQ), accompanied by enhanced proinflammatory cytokine secretion, such as tumor necrosis factor-α (TNF-α) and allo-stimulatory T-cell proliferation, as confirmed via mixed lymphocyte reactions (MLR).61 Concludingly, diverse biomaterial chemistries might contribute to the development of distinct BAMPs in terms of proteins and carbohydrates.
Compounding this, DC morphology and release of cytokines such as IL-12p40 and IL-10, are modulated via adhesive substrates-associated signaling.64 Furthermore, Archaya et al. confirmed the substrate-dependent differences in the stimulatory and costimulatory capacities of DCs derived from nonobese diabetic mice.65 Among adhesive substrates, including fibronectin, fibrinogen (Fg), collagen, vitronectin, laminin, albumin and serum, DCs cultivated on vitronectin induced the highest immunogenicity, collagen elicited the highest IL-10 secretion in DCs, and the fibronectin-coated surface gave rise to the highest level of maturation in T cells.66 However, the expression of MHC-II and CD40, cytokine secretion profiles, and capability of inducing allogeneic T-cell proliferation of DCs were independent of pre-adsorbed adhesive proteins.67 The seemingly contrasting outcomes might result from the solid substrates (Bioflex plates), which were different in nature from the TCPs used in prior studies as indicated by the authors.
In terms of biomaterial surface modification, incorporation with bioactive inorganic ions is universally viewed as a convenient, effective, and long-lasting tool to promote desired effects.68 For instance, zinc has displayed superior antibacterial and osteogenic properties in previous investigations, and its role is irreplaceable in immune responses.69,70,71 Specifically, the exogenous addition of Zn2+ induced a tolerogenic phenotype of bone marrow-derived DCs, with suppressed expression of MHC II and elevated expression of PD-L1 and PD-L2, skewing the balance of Treg/Th17 cells in favor of FoxP3+ Tregs in a model of Histoplasma capsulatum fungal infection.72 Meanwhile, Chan et al. reported that in comparison with alginate polymer-treated DCs, those encapsulated in Ca2+-crosslinked alginate presented higher levels of CD86 and MHC II while producing more inflammatory cytokine IL-1β, indicative of higher maturation levels, while Ba2+-crosslinked alginate had no such effect. Additionally, significantly upregulated IL-1β secretion was observed from calcium alginate gel-surrounded tissue in mice.73 Moreover, Schuhladen and coworkers incorporated bioactive glasses (BGs) with biologically active ions, Cu2+, Zn2+, and Cu-Zn2+, on DCs and reported that the presence of copper modulated the phenotypic performance of tolDCs in favor of T-cell polarization into an anti-inflammatory Treg milieu.74 In contrast, Li et al. proposed that γ-AlOOH mesostrands incorporated with hierarchical Cu- and Zn-buds (Cu- and Zn-AMSs) promoted BMDC maturation and proinflammatory cytokine release, which further facilitated T-cell proliferation in mice.75 Heterogeneities within bioactive ion concentrations among different types of biomaterial carriers might be plausible for the different outcomes of DC behaviors. In this context, increasing research efforts should focus on incorporating available biomaterials with biological ions in an effort to improve the effectiveness and security of their profound applications.
Surface hydrophilicity and hydrophobicity
Hydrophobicity or the hydrophobic portion of biomaterials is their inherent immunogenicity, which serves as a universal language of injury and repair.76 In contrast, hydrophilic surfaces similar to those present in natural tissue may be able to stimulate immune cells to switch activation, thus preventing a chronic immune response.77 Specifically, the wettability of the biomaterial surface determines the proteins adsorbed to it and the subsequent formation of blood clots and fibrin networks.78 However, protein adsorption on a hydrophobic surface alters its conformation through hydrophobic interactions, exposing the intrinsic domain and promoting the recruitment of host immune cells.79,80 Yang et al. fabricated a novel superamphiphilic material polycaprolactone (PCL) with an interconnected hierarchical pore structure and ascendant wettability, as confirmed by a water contact angle of 45.3°. The hydrophilic PCL displayed a facilitating effect on DC adhesion and proliferation when compared with hydrophobic surfaces. However, the authors did not further investigate the phenotype and function of DCs.81 Meanwhile, Liu et al. confirmed that poly(D,L-lactic acid) (PLA) spherical microparticles19 possessed similar size, charge, and morphology but higher hydrophobicity than PLGA or poly(monomethoxypolyethylene glycol-co-D,L-lactide) (PELA), which promoted effective antigen uptake and induced a higher level of DC maturation. In addition, adhesion force measurements further validated the strong interaction between highly hydrophobic PLA MPs and cell membranes, favoring MP internalization and the subsequent elicitation of immunity.82 Chaudhary et al. also underscored that hydrophilic surfaces preferentially modulate biological functions, including protein adsorption, cell attachment, and proliferation, thereby enhancing bioactivity and regulating biological functions.83 By immobilizing the adsorbed proteins, it is therefore promising to develop a biointerface via improvement in hydrophilicity that can promote biocompatibility and tissue regeneration.58
Surface topography, and spatial structures
Surface attributes such as porosity, roughness, three-dimensional structures, and the presence and type of micro- and nano-structures (NS) have profound influence on the phenotype and function of immune cells.42 Micro- and nano-structures have been shown to significantly affect DC behaviors.58,78 Compounding this, DCs seeded on poly(2-hydroxyethyl methacrylate) (pHEMA) and poly(dimethylsiloxane) (PDMS) scaffolds matured increasingly with decreasing pore size, with those cultivated on 20-μm pore scaffolds matured most remarkably.84 In this context, topochip, which was fabricated with polylactic acid (PLLA) or polyethylene oxide/polybutyl terephthalate (PEO/PBT), has been introduced into biomedical immunity, thereby enabling the screening of DC response to certain important biomaterial properties in a high-throughput manner.85 For instance, Unadkat et al. developed chips of PLGA with 2176 different, nonbiased, random surface patterns based on mathematical algorithms.86 Using this topochip, Kou et al. reported that a human DC-like cell line (KG-1 cells) adhered discordantly toward different topoUnits.87 Collectively, these findings highlighted that the surface topographic characteristics of biomaterials are of great significance to DC phenotype and functions and that it is beneficial to interfere with cell-biomaterial interactions by modifying surface topography in an effort to manipulate the tissue repair responses.
With most of the current studies ongoing relative to the biomaterial-surrounding milieu, the three-dimensional (3D) spatial structure, however, might better depict the microenvironment where DCs function.88 In fact, good cytocompatibility of 3D hydrogels at various concentrations was shown in terms of DC extension and surface marker expression.89 Koen van den Dries et al. reported that 3D micropatterned surfaces inhibit PGE2-mediated RhoA activation, which resulted in impaired podosome dissolution and higher MHC-II expression of DCs, suggesting the important role of three-dimensional geometry cues in regulating DC adhesive and immunomodulatory properties.90 Additionally, Daneshmandi et al. developed collagen-chitosan scaffolds to mimic the 3D microenvironment for DCs, where higher levels of maturation and cytokine production, including IL-6, IL-12, and TNF-α, were observed in comparison with those cultured on 2D culture plates. Furthermore, the induced secretion of IFN-γ and IL-4, together with the downregulated release of TGF-β, was detected during DC-T-lymphocyte cocultures in a 3D system.88 Consistently, Chen et al. reported that after exposure to pHEMA and PDMS scaffolds, the secretion of MIP-1α, IL-6 and TNF-α, along with CD86 expression on DCs, significantly increased when compared to those grown on NTCPs, indicating promotion of mDC-induced inflammation.84 Nevertheless, Sapudom et al. showed that predifferentiated mDCs presented higher levels of CCR7, MHC II, CD80, and CD86 when seeded on 2D tissue culture plastic, which were attenuated by a 3D collagen microenvironment.91 The deviations in DC maturation and function might be attributed to different cell lines as well as the intrinsic hydrophilicity and surface chemistry of the biomaterials adopted. Altogether, spatial structures may in this manner interact synergistically with other properties to yield a microenvironment favorable for tissue repair and regeneration.
Surface roughness
Roughness is another critical parameter determining immune responses, particularly for metallic implant materials. Nevertheless, it has been reported that among the multiplex properties that influence DC behavior, surface roughness might not be the critical determinant. Titanium substrates have been fabricated with typical microtopography as well as surface energy by Kou and coworkers:16 PT discs were chemically polished to be finely smooth, SLA substrates displayed a hierarchical structure of cavities with 10–50 µm indentations completely superposed by 1–2 µm pores,92 and modSLA surfaces displayed similar roughness as SLA while maintaining its high surface energy. PT- and SLA-treated DCs both displayed indistinguishably high dendritic morphology and expressed higher levels of CD86 than those cultured on TCPs. ModSLA surfaces, on the other hand, rendered DCs cultivated on them to maintain a CD86 expression level similar to iDCs and process-free immature morphology.16,17,18 Enhanced expression of proinflammatory IL-6, IL-12, and IL-18 was detected in DCs cultured on PT or SLA surfaces, while IL-1ra, IL- 4, IL-10, and TNF-α were reduced, and the outcome was the opposite in terms of modSLA surfaces.18 The characteristics of biomaterial surfaces were confirmed by assessment of surface roughness, contact angle, and surface chemistry via XPS analysis, as listed in Table 1.16,17,18,77,93,94,95,96,97 Collectively, different behaviors of DCs might be interpreted as the outcome of a higher oxygen percentage and hydrophilicity, whereas current studies have concluded that surface roughness was not decisive in DC-biomaterial interactions.17 Nevertheless, explicit effects of surface roughness on DC phenotype and function are not fully elaborated, and this prospective should be generalized in future efforts.
Surface charge
According to Gammon, the surface charge on the biomaterial surface is critical in modulating the migration and differentiation of immune cells and is generalizable across different biomaterials.59 According to Andorko et al., neutral to positive zeta potentials would induce conspicuous DC activation.98 For instance, Ma et al. evaluated DOTAP/DOPC liposome-regulated immune responses and reported that liposomes with a relatively cationic charge potently promoted DC maturation and antigen uptake, whereas low-charge liposomes failed to facilitate DC-mediated immunity even at high concentrations.99 Meanwhile, with their negative surface charge, graphene quantum dot (GQD) particles (large GQD: (−9.0 ± 1.5) mV; small GQD: (−9.4 ± 0.8) mV) significantly impaired DC-mediated allogeneic T-cell proliferation and Th1/Th17 polarization, which was in accordance with the modified secretion of IL-12p70 and altered expression of CD40, CD83, and CD86.100,101 Nevertheless, cationic materials might, on the other hand, lead to diminished activation of DCs with suppressed antigen uptake.98,102,103 In fact, investigations on the role of surface charge in DC-biomaterial interactions are ongoing, and the aforementioned attempts primarily suggest that the surface charge of biomaterials is critical in DC activation and is highly manageable, which might be a future direction in bioengineering and vaccine delivery.104,105,106
Biomedical strategies targeting DCs for modulating the balance between immunomodulation and immune reactions can significantly improve the efficacy of tissue engineering. Harnessing the surface properties (surface chemistry, hydrophilicity, topography and spatial structures, roughness, and surface charge) of biomaterials are promising to modify immune reactions while eliminating unexpected adverse reactions. Nevertheless, materials properties are generally intricate and interlace with each other, rendering what and how biomaterial properties that potentially govern DC-mediated immune responses unknown. For instance, interpretation of the varied behaviors of DCs in response towards the five commonly adopted biomaterials (agarose, alginate, chitosan, HA, and PLGA) triggered big confusions. Park indicated that hydrophobic PLGA and chitosan with highly cationic glucosamine displayed higher levels of maturation. Nevertheless, alginate, absent in hydrophobicity or negative charges, also induced higher levels of maturation within DCs in terms of TNF-α and IL-6 release.107 It is thereby reasonable to preliminarily postulate that apart from hydrophobicity and cationic charges associated with the biomaterial surface, the inherent chemical compositions, such as carbohydrate units contained in mannuronic acids and guluronic acids, might possibly determine the resultant phenotype of DCs upon biomaterial exposure. In addition, carbon dioxide and hydrocarbon contaminants introduced from the environment during film formation, and rearrangement of hydrophobic sites of biomaterial molecules during biomaterial processing procedures might, to some degree, account for the modified DC behaviors.61 To cope with the limitations in delineate the interplay between single material property and DC performance, Kou et al. performed principal component analysis (PCA), and demonstrated that titanium surface hydrophilicity and concentration of oxygen were associated with anti-inflammatory immature phenotype of DCs. Considering that these biomaterials of different forms have been ubiquitously adopted as scaffolds or cell carriers for tissue engineering,108,109,110 persistent efforts should be distributed here to delineate a comprehensive map with regard to material property-DC phenotype interactions. Fortunately, the application of immune-mediated tissue engineering has witnessed immense growth in recent decades with the development of high-throughput strategies (e.g., PCA and partial least squares regression (PLSR)).
Biomaterial-associated molecule patterns
When implanted in the host, biomaterials are capable of adsorbing or desorbing contacting proteins and form a dynamic layer of adsorbed protein, thus encouraging DCs binding and tissue repair.111,112 This layer of adhesion protein can be allogenic (derived from materials or contamination) or autologous (extra-cellular matrix (ECM) existed in blood or body fluids), and modulate host responses, such as coagulation, complement activation, and innate immunity.29,113 A recent research uncovered that biomaterial surface properties determined the adsorbed proteomic profiles and the subsequential cellular interactions.114 These recognizable motifs are referred to as ‘biomaterial associated molecule patterns’ (BAMP), which are similar with DAMP/PAMP. As first put forward by Babensee, BAMP could be interpreted as either cognate binding ligands relevant to adsorbed proteins, soluble ‘danger signals’ such as DNA, RNA, HSPs, and HMGB1, as well as intrinsic structures characteristic of biomaterials, in particular carbohydrates.115,116,117 However, the absolute conformational structures and chemical composition of BAMP have not been fully clarified. Herein, we elaborate on the connotation of BAMPs based on related studies and enumerate several BAMPs that are critical in DC-biomaterial interactions (Fig. 3).
A brief illustration of Biomaterial-associated molecular patterns. When implanted in the host, biomaterials are capable of adsorbing or desorbing contacting proteins and form a layer of adsorbed protein. Biomaterial surface properties determine the adsorbed proteomic profiles and the subsequential cellular interactions. These recognizable motifs are referred to as ‘biomaterial associated molecule patterns’, and the connotation of which could be could be summarized as three main parts: adsorbed proteins which can be allogenic (derived from materials or contamination) or autologous (ECM existed in blood or body fluids); soluble ‘danger signals’ (e.g., DNA, RNA, HMGB1, HSOs); and characteristics of the material itself
Adsorbed proteins and other macromolecules
A battery of studies has confirmed activation of DCs under exposure towards HA, up-regulated adherence to fibronectin, and induced mature phenotype treated with type 1 collagen and fibronectin.118 In addition, adhesion-limiting proteins (albumin, vitronectin, and fibronectin) have higher affinity to hydrophilic surfaces, whereas adhesion-promoting proteins (fibrinogen and IgG2) adhere easily to hydrophobic surfaces.1 Acharya et al. proposed that DC morphology and inflammatory cytokine expression (IL-12p40 and IL-10), instead of adhesion or expression of costimulatory molecules are adhesive substrate-dependent.64 Nevertheless, Lewis reported that neither surface expression of stimulatory molecules or cytokine secretion profiles were modulated via adhesive proteins, regardless of laminin, collagen, or fibrinogen.67 These seemingly contrasted results may be attributed to the different time points the two experiments undertake (24 h, 1 h).28 Compounding this, osteopontin (OPN),119 vitronectin,120 and fibronectin121 adsorbed to foreign biomaterials may be recognized by DCs and resulted in foreign body reactions (FBR).
Furthermore, Escalante and Swartzlander122,123 clarified the protein adsorption profiles on the surface of PEG hydrogels via liquid chromatography-tandem mass spectrometry. According to them, both regulators of wound healing, such as alpha-2 macroglobulin and apolipoprotein A-I, together with inflammation stimulators, including vitamin D binding protein and complement component 3 (C3), were identified at the interface. Proteins containing the bioactive motif Arg-Gly-Asp (RGD) serve as the primary cell attachment sites for a battery of adhesive ECM proteins.124,125 Identified on fibronectin,126,127 the RGD sequence has been initially recognized in numerous ECM proteins, including fibrinogen, vitronectin, laminin, and collagen.128,129,130,131 Specifically, Acharya et al. investigated the expression levels of DC maturation markers and cytokine production was enhanced in response to increased RGD peptide density, with IL-12p40 being the most sensitive marker.66,132 To better comprehend the effect of RGD in modulating the FBR, Swartzlander et al. investigated the immune response toward polyethylene glycol (PEG), PEG-RGD and PEG-RDG hydrogels, a bioincompatible peptide with similar chemistry as RGD. An equivalent profile and quantity of adsorbed proteins among these three hydrogels was observed. Conversely, inflammatory cells can recognize the adhesion peptide RGD, and significantly reduced fibrous capsule density and thickness in comparison with PEG or PEG-RDG. Their studies further verified that RGD might influence the immunogenic cellular phenotype and function without modifying the composition of the adsorbed protein layer. A possible attribution could be the retention of the intrinsic structure of proteins on PEG-RGD, which might otherwise unfold without RGD.123
Soluble danger signals released from residual cell components
DAMPs, such as HMGB1 and HSPs, not in high abundance though, were also identified.123 According to Babensee, a complicated mixture of DAMPs (such as heat shock proteins (HSP60), HSP70, HMGB1, and heparin sulfate), either as a consequence of injuries or an outcome of conserved material properties, was observed in the tissue adjacent to subcutaneously implanted PLGA scaffolds.115 It is, therefore, speculated that by binding DAMPs adsorbing to the surface of implanted biomaterial, DCs surface TLRs or CLRs might initiate biomaterial-mediated inflammation.133 In addition, Farshid et al. believed that the conformation of biomaterial-determined adsorbed proteins might lead to the emergence of various BAMPs, which was similar to an exogenous DAMP (including HSPs and HMGB1) and generate DC-mediated immune responses through integrins and PRRs.134 However, this interpretation should be viewed with caution and substantiated experimentally.
High mobility group box 1 (HMGB1)
The axis high mobility group box 1 (HMGB1) and the Receptor for Advanced Glycation End Products (RAGE) are related to inflammatory and healing events, with HMGB1 being the prototypical and well-characterized DAMP.135 Considering this, molecular dynamics computer simulations have been introduced to explore the content and conformation of HMGB1 induced at the surface of titanium.136 Biguetti reported that the inhibition of HMGB1 or RAGE promoted inflammatory cell infiltration, impaired osseointegration, and affected organic and mineralized bone matrix dynamics.137 Nevertheless, questions remain as to whether the enhanced HMGB1 detected in the implantation site is a consequence of injuries that prime the immune system, or an outcome of biomaterial intrinsic properties. To further elucidate the specific constitution, Bennewitz performed immunoblotting analysis on the tissue exudates surrounding implanted PLGA scaffolds and injected PLGA MPs.138 In addition to the intragroup heterogeneity arising from tissue damage which increased the concentration of DAMPs, higher levels of HMGB1 were detected around PLGA materials, signaling the potential role of HMGB1 in DC-biomaterial interactions.
Heat shock proteins (HSPs)
HSPs are a family of conserved chaperone molecules that prevent aggregation and misfolding of nascent polypeptides while expediting protein folding, thereby maintaining cellular functions.139 HSPs (e.g., HSP 70A, 70B, 9,0 and 47) expressions were modulated by the hydrophilicity of the biomaterial,140 which have been involved in the host immune response via regulating the expression of TLR2 and TLR4.141 Babensee concluded that HSPs can probably link biomaterial-mediated cell stress or necrosis and the induction of innate immunity at implant sites.115 Having confirmed the presence of HSP60 within the substrates, McKiel also observed increased NF-κB/AP-1-dependent SEAP activity on poly(methyl methacrylate) (PMMA) and polydimethylsiloxane (PDMS) with 10% FBS, which induced the expression of proinflammatory cytokines as compared to those preadsorbed with serum only. They speculated that inflammatory cellular function and immunogenic responses rely highly on the biomaterial-directed protein adsorption.
Conserved structures characteristic of the material itself
Intrinsic structures characteristic of biomaterials (e.g., surface chemistry, hydrophilicity) determined inflammatory cellular interactions, in particular DCs, and the subsequential immune reactions. For instance, when an implanted hydrophobic biomaterial interact with blood, proteins may instantaneously adsorb, generally through hydrophobic interactions.142 In contrast, hydrophilic biomaterials indirectly generate an interfacial free energy of low value with biological fluids.143 Hasek uncovered multiple-layer protein adsorption profiles on the surfaces of PEG hydrogels in vivo, as a consequence of hydrophobic interactions within polyacrylate chains144 or through hydrogen bonding amid PEG and amide groups contained in the polypeptide mainstay of the proteins.145
Carbohydrates at the DC-biomaterial interface, as a key component of the adsorbed protein or an intrinsic material structures, are prominent in balancing the biocompatibility and biodegradability of biomaterials.146 Considering the evidenced linkage between altered glycosylation and disease progression,147 it is speculated that biomaterials may display analogous segments to migrating DCs if the adsorbed proteins are conformationally altered and present a modified carbohydrate profile.148 To confirm the existence of analogous BAMPs in the immunogenic cellular-biomaterial interface, Shankar and coworkers conducted enzyme-linked lectin assays (ELLA) to probe for carbohydrate ligands of CLRs on self-assembled monolayers (SAMs) delivering -OH, -CH3, -COOH or -NH2. They observed higher α-galactose on COOH SAMs and higher mannose on NH2 SAMs, indicating that unique presentation of associated carbohydrates at the biomaterial interface mediates the interactions of the innate immune response.149,150
To our knowledge, however, efforts to systematically characterize the molecular composition of BAMPs have just set off, and the mechanisms by which DCs recognize and react to biomaterials have not yet been fully elucidated. To better identify the adsorbed protein profiles and involved posttranslational modifications, including carbohydrates and lipids, sophisticated tools (mass spectrometry (MS),151 SDS-PAGE and immunoblot analysis152) and statistical modeling (PCA,16,153,154 PLSR,155 high-throughput nanoimmunoassay chip,156 single-cell analysis157) have been introduced. Hopefully, extensive interpretation of the immune events involved in the formation of adsorbed proteins and biomaterial recognition might provide insights for the development of next generation of biomaterials.158
Mechanisms underlying dendritic cells response to biomaterials
Integrins
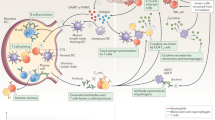
Integrins refer to heterodimeric type I transmembrane proteins consisting of α and β subunits, which also constitute one of the major intracellular receptors that modulate cellular survival, proliferation, and differentiation.1,159,160 Random permutation of subfamilies of α and β subunits contributes to the formation of 24 distinct types of integrins, and the partnering of different subunit chains confers variable function to the receptor.1 Among them, leukocytes uniquely express the β2 subfamily of integrins, including αLβ2 (CD11a), αMβ2 (CD11b), αXβ2 (CD11c), and αDβ2 (CD11d). Moreover, a battery of immune events are regulated via β2 integrins: recruitment to sites of inflammation,161 intracellular contact162 and signaling.163 Rogers et al. observed that β2 integrins in DC adhesive podosomes are at the biomaterial interface and in direct contact with TCPs and PLGA films, and DCs treated with anti-β2 integrin antibody expressed lower levels of CD86. In this regard, their study demonstrated that a basal level of β2 signaling is a prerequisite to manipulate DC adhesion and maintain maturation in response to biomaterials.164 Similarly, Farshid et al. postulated that DC adhesion to the biomaterial via integrins correlated highly with DC maturation, which in turn determined the immune responses.1 Having reviewed published work concerning the role of DC integrin in biomaterials, Franz speculated that integrin signaling might serve as an alternative mechanism of DC activation to PRR engagement.165
Integrins are the initial adhering receptors for ECM proteins, thus enabling the nuclear transcription factor-kappa B (NF-κB) and Jak/STAT signaling pathways,166 the former of which is considered a critical mediator of DC maturation molecules, including CD80 and CD86.167 However, the intracellular bonding between integrin engagement and DC maturation is controversial. DCs treated with PLGA films after 24 h did not necessarily reveal enhanced expression of translational NF-κB.168 Considering this, one possible explanation indicated by Rogers was that adhesion alone was insufficient for DC activation, which merely allowed for the localization of other coreceptors for engagement.164 It is reasonable, in this context, to assume coreceptors other than integrins, such as TLRs and CLRs, to be effective targets associated with DC-biomaterial interactions, the role of which will be elaborated below (Fig. 4).
Mechanisms underlying dendritic cells response to biomaterials. a The mechanism of integrins. β2 integrins are virtually the first receptors adhering to the ECM proteins, thus initiating NF-kB signaling pathways, which potentiates multiple inflammatory events. b The mechanism of TLRs, especially of TLR 2/4/6. TLR 2/4/6 signals through MyD88 or TRIF respectively, which terminated at two events: (i) activates the NF-kB-mediated regulation of inflammatory (like TNF-α or IL-6) and immunoregulatory genes (like TGF-β or IL-10); (ii) phosphorylates IRF3 and IRF7, leading to the consequential expression of co-stimulatory molecules such as CD80 and CD86 and production of type I interferons (e.g., IFN-α and IFN-β). c The mechanism of CLRs. DC-SIGN and CD205 are among the most efficient CLRs that enhance antigen uptake and presentation by DCs. d The mechanism of inflammasomes, especially of NLRP3. In the presence of NF-kB, cytosolic NLRP3 can also be stimulated via biomaterial-derived signals and forms an intracellular multiprotein complex that catalyzes the conversion of caspase-1, thus regulating the production of highly inflammatory cytokines such as IL-1β and IL-18. e The mechanism of autophagy. DCs in the presence of certain biomaterials express high levels of ILT3 and PDL1, as characteristic co-inhibitory molecules of tolDC, while upregulate transcriptional profile of ATGs such as ATF4 and FOXO3
Toll-like receptors
TLRs with leucine-rich repeats in their ectodomains169,170 are PRRs that bind PAMPs derived from pathogens and DAMPs from dying or injured cells,171 the implication of which has been extended to BAMPs.115 Thirteen subtypes of TLRs have been reported, and among them, a retro-viral insertion has inactivated TLR10 in mice,172 whereas TLR11, −12, and −13 do not occur in humans.173 TLR1, −2, −4, −5, −6, and −10 are presented at the cell membrane, which mainly recognize microbial components, including lipids, proteins, and lipoproteins, while TLR3, −7, −8, −9, −11, −12, and −13 are expressed in the endosome.174,175 All TLRs signal through myeloid differentiation primary-response protein 88 (MyD88), a crucial adaptor molecule recruited upon binding of TLRs, DAMPs and BAMPs,176 except for TLR3, which confers to the TIR domain containing adapter-inducing interferon-beta (TRIF), and TLR4 through both MyD88 and TRIF.177 The network map of TLRs has been delineated substantially owing to everlasting efforts, which terminated at two events: (1) potentiates the transcription factor NF-κB178,179 which are involved in the regulation of many proinflammatory and immunoregulatory genes;180,181 (2) phosphorylates IFN regulatory factor 3 (IRF3) as well as IRF7,1 resulting in the consequential costimulatory molecules expression and type I interferon secretions, such as IFN-α and IFN-β.175,182
Apart from the large body of work exploring macrophage-induced immune responses toward orthopedic metallic implants,177 biomedical polymers,183,184,185,186 nanoparticles, and microparticles,187 the involvement of TLR signaling is progressively implicated in DC-biomaterial interactions. TLR4 serves as one of the most potent PRRs188 and is considered the core receptor in the recognition of ligands, including LPS,189 HSP60 and 70,141 HMGB1,190 hyaluronan,191 and fibrinogen.192 A previous investigation by Rogers and colleagues revealed that once implanted intraperitoneally in TLR4-knockout mice for 16 h, PET discs triggered an altered infiltration of leukocytes, which shifted from equivalently neutrophils and monocytes/macrophages to predominantly neutrophils, suggesting the critical role of TLR4 in the primary recognition of biomaterials.171 Nevertheless, no differences could be discerned in regard to the thickness of fibrous encapsulation encompassing the PET discs at 14 days, indicating that the impact of TLR4 was confined to the acute phase in response to the implanted biomaterial. In contrast, another study in C57BL6-TLR4-/- mice reported less inflammatory cell infiltration and angiogenesis around the silicone prosthesis and a 1.96-fold thicker capsule around the implant in the absence of TLR4 at Day 14, pointing out the drastic effect of TLR4 in the host response to silicone implants.193 Between the two opposite findings, the preadsorbed fibrinogen might account for the different extent to which immune reactions are exacerbated, and it coincides with and broadens our previous postulation that the adsorbed protein layer might mediate fibrous encapsulation and the subsequent temporal immune response via TLR4.186 By MyD88-deficient DCs, Shokouhi et al. clarified that complicated TLR/MyD88- signaling (particularly TLR2, TLR4, and TLR6) was involved in DC-biomaterial interactions, leading to the activation and maturation of DCs and conferring on them the ability to switch on antigen-specific T cells.31 To further clarify the influence of TLR4/MyD88 signaling in DC-mediated biomaterial recognition, Uto et al. incubated murine BMDCs with biodegradable nanoparticles (NPs) supplemented with poly(g-glutamic acid) (g-PGA). Significant enhancement regarding costimulatory marker expression (e.g., CD40, CD80, and CD86) and cytokine release (e.g., TNF-α, IL-6, and IL-12) was detected, which were probably mediated through phosphorylated forms of p38, SAPK/JNK, and ERK. Nevertheless, consistent trends were not observed in MyD88−/− or TLR4−/− mice.194 Collectively, their work elucidated that biomaterial-triggered DCs that functioned predominantly via TLR4-MyD88-MAPK signaling pathways.
C-type lectin receptors
CLRs strongly participate in the immune response and biomaterial recognition,195 representing a family of receptors, such as collectins, selectins, lymphocyte lectins, and proteoglycans.196 Located on the surface of DCs, CLRs ligate to the carbohydrate structures of pathogens, self-antigens and biomaterials.115,197 DC-specific intracellular adhesion molecule-3 grabbing nonintegrin (DC-SIGN) recognizes mannose-containing structures as well as Lewis antigens (Le(a), Le(b), Le(x), and Le(y)).198 By exploiting DC-SIGN ligand multivalency, internalization, lysosomal trafficking, antigen presentation and cytokine release could be optimized with glycopeptide polymers Le(b)-conjugated poly(amido amine) (PAMAM).199 Additionally, the notable feature of CD205 to facilitate DC antigen uptake and presentation in an exceptionally efficient and specific manner has rendered it one of the research focuses. Specifically, CD205 could be a bilateral mediator, either immunogenic or immunotolerant, depending on the maturation level and the expression of stimulatory signals.200,201
CLR recognition is influenced by the glycosylation of the carbohydrate recognition domain (CRD) region in dendritic cell immunoreceptor (DCIR) via its immunoreceptor tyrosine-based inhibitory motif (ITIM). Currently, researchers have been devoted to clarifying the role of CLRs in DC-biomaterial interactions and in this manner shed novel light on engineering cellular function and modulating immunity. Hotaling et al. showed that as the cationization level of conjugates or glycan density increased and so did nonspecific lectin binding. Once blocked with EDTA or specific antibodies for DC-SIGN or Dectin-1, antigen internalization and maturation of DCs was inhibited.158,202,203 Interestingly, the interplay of CLRs and TLRs in DCs could possibly result either in the onset of inflammatory reactions or in tolerance maintenance by the defense system.204,205 Nevertheless, studies concerning the collaborative role of CLRs and TLRs are rare and call for future efforts.
Inflammasomes
Inflammasomes refer to a family of cytosolic multiprotein complexes206 and play a critical role in pathogen clearance, adjuvant activity, and overall immunity.207 Upon binding with NOD-like receptors (NLRs),206,208 inflammasomes function through activation of caspase-1, 4, 5, or 11 and cleavage of cytokines, including IL-1β and IL-18, followed by pyroptosis.206 Among the well-understood inflammasomes, NLRP3 is effective in antitumor immunity and vaccination responses induced by DCs.209,210,211 However, abnormal inflammasome activation has been associated with numerous autoimmune diseases.212 Therefore, some researchers have attempted to tune inflammasome-mediated immunomodulation by harnessing the properties of implanted biomaterials in an attempt to facilitate future applications in immunotherapies. Li et al. found that NLRP3 played a substantial role in mesoporous silica microrod (MSR) scaffold-induced BMDC IL-1β secretion in vitro as well as immune cell trafficking in vivo.213 Most DCs recruited to scaffolds in NLRP3−/− mice exhibited an immature phenotype when compared to WT mice.213 Sharp et al. demonstrated that uptake of PLG microparticles by DCs dramatically accelerated IL-1β secretion and caspase-1 activation, which depended on the NLRP3 inflammasome, yet the production of antigen-specific antibodies was not influenced.214 Similarly, another study also observed that the immunogenicity of peptide nanofibers was not necessarily accompanied by the production of IL-1β or caspase-1/11. Their findings confirmed that activation of the NLRP3 inflammasome might not be a decisive factor involved in T-cell-mediated immune responses against these materials.215
To elucidate the underlying mechanism, previous studies reported that uptake of microparticles could induce lysosomal damage,214 and DAMPs generated during this process were recognized by NLRP3.216 Upon activation, NLRP3 forms an intracellular multiprotein complex that manipulates cytokine release (e.g., IL-1β and IL-18), leading to the infiltration of inflammatory cells for cleavage of the local particulates.213,216 Li et al. also considered that a similar pathway might function in terms of host immunity toward MSR scaffolds,213 and lysosomal perturbation might be a fundamental regulator of NLRP3 inflammasome activation.216,217 As Saikat et al. indicated, lysosomal rupture is an initial step in inflammasome activation, which then precedes other pathways.217
mTOR-NF-kB mechanism involved in autophagy
Autophagy refers to a lysosomal pathway for cellular homeostasis control, which is presently known to be the signal light of immune cells, orchestrating their proliferation, cytokine secretion, and survival. Reactive oxygen species (ROS) serve as crucial regulators of oxidative stress and redox signaling in immune cells,218 the inhibition of which is involved in the tolerogenic phenotype and allo-stimulatory capacity of DCs.101 Nevertheless, the involvement of ROS in the manipulation of DC-mediated immunity remains to be explicitly examined, considering some opposite findings.219 In addition, mammalian target of rapamycin (mTOR) is a decisive mediator of cellular metabolism, NF-κB translocation, phenotypic conversion, antigen presentation, and T-cell activation of DCs.220,221,222 Autophagy is thus mediated by mTOR inhibition-induced activation of Atg1/Unc-51-like autophagy activating kinase 1 (ULK1) and subsequent posttranslational modifications of autophagy-related genes (ATGs).223
The role of autophagy in immunogenic function and cytokine production upon exposure to biomaterials was explored in monocytes and macrophages.224,225,226 A recent study revealed that DCs in contact with GQD presented upregulated ILT3 as well as PDL1, characteristic coinhibitory molecules of tolDCs, and were capable of potentiating allogeneic CD4+CD25hiFoxP3+ Treg cells. Autophagic turnover (flux) was enhanced in GQD-treated DCs, as evidenced by the upregulated transition from microtubule-related LC3-I to autophagosome-associated LC3-II and the upregulated transcriptional profile of ATGs, such as ATF4, FOXO1, and FOXO3. Most notably, genetic suppression of autophagy via RNA interference (RNAi) techniques undermined the protolerogenic effects of GQD on DCs.101 Consistently, the delivery of the autophagy inhibitor chloroquine enhanced p62 and LC3-II expression and reduced IL-12p70 release in human monocyte-derived DCs in a p38-dependent manner, thereby promoting Th17-mediated persistent inflammation.227
Conclusions and future perspectives
Novel understanding of immune responses towards implanted biomaterials can excitingly help tackle the challenges of protracted inflammation or immune-mediated rejection, by harnessing the specificity of immune cells-biomaterial interactions. During the process, immunocytes are often recruited consecutively in an organized manner in the context of foreign biomaterials, with DCs playing an orchestrating role. Within the last decade, DC-mediated immune responses toward implanted biomaterials have been demonstrated to be diverse according to their physiochemical properties, the comprehensive interpretation of which may benefit future vaccination, cancer therapy, and the design and manufacture of novel biomedical materials. However, challenges remain. First, the new paradigm in biomaterials research calls for the interaction with the immune system instead of simply suppressing it. Considering this, incorporation with bioactive inorganic ions is universally viewed as a convenient, effective and long-lasting tool to promote desired effects. Nevertheless, their regulatory effects on DCs remain not fully elucidated. Herein, increasing research efforts should focus on incorporating available biomaterials with biological ions, in an effort to improve the effectiveness and security of their profound applications. Harnessing DC-mediated immune microenvironment for material rejection or integration is promising. Biomaterials carrying antigens or delivering vaccines can enhance host immune reactions and aid in anti-tumor therapy, while dampening immune responses by surface property modifications will assist in autoimmunity and tissue engineering. One major obstacle here in elucidating the immunogenic effects of material properties lies in its multifactorial features. To cope with this, persistent efforts should be distributed here to delineate a comprehensive map with regard to material property-DC performance interactions. Fortunately, the application of immune-mediated tissue engineering has witnessed immense growth in recent decades with the development of high-throughput strategies (e.g., PCA and PLSR). Furthermore, BAMP serve as the key immunogenic cue associated with DC-biomaterial interactions. To our knowledge, however, efforts to systematically characterize the molecular composition of BAMPs have just set off, and the mechanisms by which DCs recognize and react to biomaterials have not yet been fully elucidated. To extensively identify the adsorbed protein profiles and involved posttranslational modifications, including carbohydrates and lipids, sophisticated tools and statistical modeling have been introduced, which might provide insights for the development of next generation of biomaterials. In addition, spatiotemporal activation of dendritic cells and their precise modulation mechanism can provide insights for future development of novel biomaterials, and such approaches have not been well explored in the tissue engineering community. It is postulated that multiple receptors might be associated with DC-biomaterial interactions collaboratively. Better understanding towards the underlying mechanisms could pave the way for the rational design and clinical application of material-based strategies with desired immune effects. Notably, the immunologic microenvironment is rather complex and rapidly changeable, while the majority of the work in this field relying on in vitro studies is less convincing. Compounding this, the remarkable advances in cell biology and biomaterials science and engineering also add to the uncertainty and complexity. Therefore, much work still needs to be done to unveil the behavior and biofunction of DCs toward novel implanted biomaterials in vivo.
References
Eslami-Kaliji, F., Sarafbidabad, M., Rajadas, J. & Mohammadi, M. R. Dendritic cells as targets for biomaterial-based immunomodulation. ACS Biomater. Sci. Eng. 6, 2726–2739 (2020).
Franz, S., Rammelt, S., Scharnweber, D. & Simon, J. C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 32, 6692–6709 (2011).
Van Brussel, I., Berneman, Z. N. & Cools, N. Optimizing dendritic cell-based immunotherapy: tackling the complexity of different arms of the immune system. Mediators Inflamm. 2012, 690643 (2012).
Denzer, K. et al. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J. Immunol. 165, 1259–1265 (2000).
Collin, M., McGovern, N. & Haniffa, M. Human dendritic cell subsets. Immunology 140, 22–30 (2013).
Collin, M. & Bigley, V. Human dendritic cell subsets: an update. Immunology 154, 3–20 (2018).
Bar-On, L. & Jung, S. Defining dendritic cells by conditional and constitutive cell ablation. Immunol. Rev. 234, 76–89 (2010).
Ohnmacht, C. et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J. Exp. Med. 206, 549–559 (2009).
Manicassamy, S. & Pulendran, B. Dendritic cell control of tolerogenic responses. Immunol. Rev. 241, 206–227 (2011).
Constantino, J., Gomes, C., Falcão, A., Neves, B. M. & Cruz, M. T. Dendritic cell-based immunotherapy: a basic review and recent advances. Immunol. Res. 65, 798–810 (2017).
Worbs, T., Hammerschmidt, S. I. & Förster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 17, 30–48 (2017).
Morelli, A. E., Hackstein, H. & Thomson, A. W. Potential of tolerogenic dendritic cells for transplantation. Semin. Immunol. 13, 323–335 (2001).
Waisman, A., Lukas, D., Clausen, B. E. & Yogev, N. Dendritic cells as gatekeepers of tolerance. Semin. Immunopathol. 39, 153–163 (2017).
Yin, X., Chen, S. & Eisenbarth, S. C. Dendritic cell regulation of T helper cells. Annu. Rev. Immunol. 39, 759–790 (2021).
Sadtler, K. et al. Proteomic composition and immunomodulatory properties of urinary bladder matrix scaffolds in homeostasis and injury. Semin. Immunol. 29, 14–23 (2017).
Kou, P. M., Schwartz, Z., Boyan, B. D. & Babensee, J. E. Dendritic cell responses to surface properties of clinical titanium surfaces. Acta Biomater. 7, 1354–1363 (2011).
Zheng, X., Zhou, F., Gu, Y., Duan, X. & Mo, A. Effect of different titanium surfaces on maturation of murine bone marrow-derived dendritic cells. Sci. Rep. 7, 41945 (2017).
Yang, Y., Wang, X., Miron, R. J. & Zhang, X. The interactions of dendritic cells with osteoblasts on titanium surfaces: an in vitro investigation. Clin. Oral. Investig. 23, 4133–4143 (2019).
Moreau, A. et al. Tolerogenic dendritic cells actively inhibit T cells through heme oxygenase-1 in rodents and in nonhuman primates. FASEB J. 23, 3070–3077 (2009).
Fu, H. et al. Dendritic cells transduced with SOCS1 gene exhibit regulatory DC properties and prolong allograft survival. Cell Mol. Immunol. 6, 87–95 (2009).
Fu, C. L., Chuang, Y. H., Huang, H. Y. & Chiang, B. L. Induction of IL-10 producing CD4+T cells with regulatory activities by stimulation with IL-10 gene-modified bone marrow derived dendritic cells. Clin. Exp. Immunol. 153, 258–268 (2008).
Levings, M. K. et al. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+Tr cells. Blood 105, 1162–1169 (2005).
Széles, L. et al. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J. Immunol. 182, 2074–2083 (2009).
van der Aar, A. M. et al. Vitamin D3 targets epidermal and dermal dendritic cells for induction of distinct regulatory T cells. J. Allergy Clin. Immunol. 127, 1532–1540.e1537 (2011).
Yan, X., Zhou, M., Yu, S., Jin, Z. & Zhao, K. An overview of biodegradable nanomaterials and applications in vaccines. Vaccine 38, 1096–1104 (2020).
Thomson, A. W. Tolerogenic dendritic cells: all present and correct. Am. J. Transpl. 10, 214–219 (2010).
Raker, V. K., Domogalla, M. P. & Steinbrink, K. Tolerogenic dendritic cells for regulatory T cell induction in man. Front. Immunol. 6, 569 (2015).
Zhu, F. J., Tong, Y. L., Sheng, Z. Y. & Yao, Y. M. Role of dendritic cells in the host response to biomaterials and their signaling pathways. Acta Biomater. 94, 132–144 (2019).
Pacelli, S. et al. Tailoring biomaterial surface properties to modulate host-implant interactions: implication in cardiovascular and bone therapy. J. Mater. Chem. B 4, 1586–1599 (2016).
Abaricia, J. O. et al. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 133, 58–73 (2021).
Shokouhi, B. et al. The role of multiple toll-like receptor signalling cascades on interactions between biomedical polymers and dendritic cells. Biomaterials 31, 5759–5771 (2010).
Leifer, C. A. Dendritic cells in host response to biologic scaffolds. Semin. Immunol. 29, 41–48 (2017).
Lopresti, S. T. & Brown, B. N. Host response to naturally derived biomaterials-ScienceDirect. In (ed Stephen F. Badylak) Host Response Biomater., 53–79 (Academic Press; 2015).
Anderson, J. M. Biological Responses to Materials. Annu. Rev. Mater. Res. 31, 81–110 (2001).
Doloff, J. C. et al. Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates. Nat. Mater. 16, 671–680 (2017).
Kamath, A. T. et al. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J. Immunol. 165, 6762–6770 (2000).
Madel, M. B. et al. Immune function and diversity of osteoclasts in normal and pathological conditions. Front. Immunol. 10, 1408 (2019).
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Devi, K. S. & Anandasabapathy, N. The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues. Semin. Immunopathol. 39, 137–152 (2017).
Schlitzer, A., McGovern, N. & Ginhoux, F. Dendritic cells and monocyte-derived cells: Two complementary and integrated functional systems. Semin. Cell Dev. Biol. 41, 9–22 (2015).
Steinman, R. M. & Banchereau, J. Taking dendritic cells into medicine. Nature 449, 419–426 (2007).
Rostam, H. M., Singh, S., Vrana, N. E., Alexander, M. R. & Ghaemmaghami, A. M. Impact of surface chemistry and topography on the function of antigen presenting cells. Biomater. Sci. 3, 424–441 (2015).
Barone & William, R. Host Response to Biomaterials for Pelvic Floor Reconstruction. (Elsevier Inc., 2015).
Yoshida, M., Mata, J. & Babensee, J. E. Effect of poly(lactic-co-glycolic acid) contact on maturation of murine bone marrow-derived dendritic cells. J. Biomed. Mater. Res. A 80, 7–12 (2007).
Riol-Blanco, L. et al. The chemokine receptor CCR7 activates in dendritic cells two signaling modules that independently regulate chemotaxis and migratory speed. J. Immunol. 174, 4070–4080 (2005).
Heuzé, M. L. et al. Migration of dendritic cells: physical principles, molecular mechanisms, and functional implications. Immunol. Rev. 256, 240–254 (2013).
Hotchkiss, K. M., Clark, N. M. & Olivares-Navarrete, R. Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations. Biomaterials 182, 202–215 (2018).
Wculek, S. K. et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2020).
Morelli, A. E. & Thomson, A. W. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat. Rev. Immunol. 7, 610–621 (2007).
Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Roncarolo, M. G. & Battaglia, M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat. Rev. Immunol. 7, 585–598 (2007).
Steinman, R. M. The control of immunity and tolerance by dendritic cell. Pathol. Biol. 51, 59–60 (2003).
Gleisner, M. A., Rosemblatt, M., Fierro, J. A. & Bono, M. R. Delivery of alloantigens via apoptotic cells generates dendritic cells with an immature tolerogenic phenotype. Transpl. Proc. 43, 2325–2333 (2011).
Steinman, R. M., Turley, S., Mellman, I. & Inaba, K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med 191, 411–416 (2000).
Lewis, J. S. & Keselowsky, B. G. Role of dendritic cells in response to biomaterials. In (ed Stephen F. Badylak) Host Response Biomater., 131–150 (Academic Press; 2015).
Tai, Y., Wang, Q., Korner, H., Zhang, L. & Wei, W. Molecular mechanisms of T cells activation by dendritic cells in autoimmune Diseases. Front Pharm. 9, 642 (2018).
Watanabe, N. et al. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 436, 1181–1185 (2005).
Batool, F. et al. Modulation of immune-inflammatory responses through surface modifications of biomaterials to promote bone healing and regeneration. J. Tissue Eng. 12, 20417314211041428 (2021).
Gammon, J. M. & Jewell, C. M. Engineering immune tolerance with biomaterials. Adv. Health. Mater. 8, e1801419 (2019).
Wei, F. et al. Host response to biomaterials for cartilage tissue engineering: key to remodeling. Front. Bioeng. Biotechnol. 9, 664592 (2021).
Park, J. & Babensee, J. E. Differential functional effects of biomaterials on dendritic cell maturation. Acta Biomater. 8, 3606–3617 (2012).
Babensee, J. E. & Paranjpe, A. Differential levels of dendritic cell maturation on different biomaterials used in combination products. J. Biomed. Mater. Res A 74, 503–510 (2005).
Yoshida, M. & Babensee, J. E. Differential effects of agarose and poly(lactic-co-glycolic acid) on dendritic cell maturation. J. Biomed. Mater. Res. A 79, 393–408 (2006).
Acharya, A. P., Dolgova, N. V., Clare-Salzler, M. J. & Keselowsky, B. G. Adhesive substrate-modulation of adaptive immune responses. Biomaterials 29, 4736–4750 (2008).
Hof, M. et al. Esthetic evaluation of single-tooth implants in the anterior mandible. Clin. Oral. Implants Res. 25, 1022–1026 (2014).
Acharya, A. P., Dolgova, N. V., Xia, C. Q., Clare-Salzler, M. J. & Keselowsky, B. G. Adhesive substrates modulate the activation and stimulatory capacity of non-obese diabetic mouse-derived dendritic cells. Acta Biomater. 7, 180–192 (2011).
Lewis, J. S. et al. The effect of cyclic mechanical strain on activation of dendritic cells cultured on adhesive substrates. Biomaterials 34, 9063–9070 (2013).
Zreiqat, H. et al. The incorporation of strontium and zinc into a calcium-silicon ceramic for bone tissue engineering. Biomaterials 31, 3175–3184 (2010).
Su, Y. et al. Zinc-based biomaterials for regeneration and therapy. Trends Biotechnol. 37, 428–441 (2019).
Cuozzo, R. C. et al. Biological evaluation of zinc-containing calcium alginate-hydroxyapatite composite microspheres for bone regeneration. J. Biomed. Mater. Res B Appl Biomater. 108, 2610–2620 (2020).
Li, Y. et al. Additively manufactured biodegradable porous zinc. Acta Biomater. 101, 609–623 (2020).
George, M. M., Subramanian Vignesh, K., Landero Figueroa, J. A., Caruso, J. A. & Deepe, G. S. Jr. Zinc induces dendritic cell tolerogenic Phenotype and Skews Regulatory T Cell-Th17 balance. J. Immunol. 197, 1864–1876 (2016).
Chan, G. & Mooney, D. J. Ca(2+) released from calcium alginate gels can promote inflammatory responses in vitro and in vivo. Acta Biomater. 9, 9281–9291 (2013).
Schuhladen, K. et al. Cu, Zn doped borate bioactive glasses: antibacterial efficacy and dose-dependent in vitro modulation of murine dendritic cells. Biomater. Sci. 8, 2143–2155 (2020).
Li, X. et al. Hierarchically porous, and Cu- and Zn-containing γ-AlOOH mesostrands as adjuvants for cancer immunotherapy. Sci. Rep. 7, 16749 (2017).
Seong, S. Y. & Matzinger, P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 4, 469–478 (2004).
Hotchkiss, K. M. et al. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 31, 425–434 (2016).
Kopf, B. S., Ruch, S., Berner, S., Spencer, N. D. & Maniura-Weber, K. The role of nanostructures and hydrophilicity in osseointegration: In-vitro protein-adsorption and blood-interaction studies. J. Biomed. Mater. Res. A 103, 2661–2672 (2015).
Lu, D. R. & Park, K. Effect of surface hydrophobicity on the conformational changes of adsorbed fibrinogen. J. Colloid Interface 144, 271–281 (1991).
Heuberger, M., Drobek, T. & Spencer, N. D. Interaction forces and morphology of a protein-resistant poly(ethylene glycol) layer. Biophys. J. 88, 495–504 (2005).
Yang, K., Du, J., Zhang, Z., Liu, D. & Ren, T. Facile and eco-friendly preparation of super-amphiphilic porous polycaprolactone. J. Colloid Interface Sci. 560, 795–801 (2020).
Liu, Y. et al. Surface hydrophobicity of microparticles modulates adjuvanticity. J. Mater. Chem. B 1, 3888–3896 (2013).
Chaudhary, P., Pesacreta, T. C. & Misra, R. D. Interplay between protein-modified surface and functional response of osteoblasts. J. Biomed. Mater. Res A 100, 3157–3166 (2012).
Chen, R., Ma, H., Zhang, L. & Bryers, J. D. Precision-porous templated scaffolds of varying pore size drive dendritic cell activation. Biotechnol. Bioeng. 115, 1086–1095 (2018).
Meredith, J. C. et al. Combinatorial characterization of cell interactions with polymer surfaces. J. Biomed. Mater. Res A 66, 483–490 (2003).
Unadkat, H. V. et al. An algorithm-based topographical biomaterials library to instruct cell fate. Proc. Natl Acad. Sci. USA 108, 16565–16570 (2011).
Kou, P. M. Elucidation of dendritic cell response-material property relationships using high-throughput methodologies. Dissertations & Theses–Gradworks (2011).
Daneshmandi, S., Dibazar, S. P. & Fateh, S. Effects of 3-dimensional culture conditions (collagen-chitosan nano-scaffolds) on maturation of dendritic cells and their capacity to interact with T-lymphocytes. J. Immunotoxicol. 13, 235–242 (2016).
Groell, F., Kalia, Y. N., Jordan, O. & Borchard, G. Hydrogels in three-dimensional dendritic cell (MUTZ-3) culture as a scaffold to mimic human immuno competent subcutaneous tissue. Int J. Pharm. 544, 297–303 (2018).
van den Dries, K. et al. Geometry sensing by dendritic cells dictates spatial organization and PGE(2)-induced dissolution of podosomes. Cell Mol. Life Sci. 69, 1889–1901 (2012).
Sapudom, J. et al. Dendritic cell immune potency on 2D and in 3D collagen matrices. Biomater. Sci. 8, 5106–5120 (2020).
Rupp, F. et al. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res A 76, 323–334 (2006).
Buser, D. et al. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 83, 529–533 (2004).
Zhao, G. et al. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. A 74, 49–58 (2005).
Schwarz, F. et al. Potential of chemically modified hydrophilic surface characteristics to support tissue integration of titanium dental implants. J. Biomed. Mater. Res B Appl Biomater. 88, 544–557 (2009).
Alfarsi, M. A., Hamlet, S. M. & Ivanovski, S. Titanium surface hydrophilicity enhances platelet activation. Dent. Mater. J. 33, 749–756 (2014).
Park, J., Gerber, M. H. & Babensee, J. E. Phenotype and polarization of autologous T cells by biomaterial-treated dendritic cells. J. Biomed. Mater. Res A 103, 170–184 (2015).
Andorko, J. I., Pineault, K. G. & Jewell, C. M. Impact of molecular weight on the intrinsic immunogenic activity of poly(beta amino) esters. J. Biomed. Mater. Res. A 105, 1219–1229 (2017).
Ma, Y. et al. The role of surface charge density in cationic liposome-promoted dendritic cell maturation and vaccine-induced immune responses. Nanoscale 3, 2307–2314 (2011).
Terhune, J., Berk, E. & Czerniecki, B. J. Dendritic cell-induced Th1 and Th17 cell differentiation for cancer therapy. Vaccines 1, 527–549 (2013).
Tomić, S. et al. Graphene quantum dots suppress proinflammatory T cell responses via autophagy-dependent induction of tolerogenic dendritic cells. Biomaterials 146, 13–28 (2017).
Verma, A. & Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 6, 12–21 (2010).
Silva, A. L. et al. Nanoparticle impact on innate immune cell pattern-recognition receptors and inflammasomes activation. Semin. Immunol. 34, 3–24 (2017).
Vitor, M. T. et al. Cationic liposomes produced via ethanol injection method for dendritic cell therapy. J. Liposome Res. 27, 249–263 (2017).
Cong, X. et al. Cationic Liposome/DNA complexes mediate antitumor immunotherapy by promoting immunogenic tumor cell death and dendritic cell activation. ACS Appl Mater. Interfaces 12, 28047–28056 (2020).
Ni, Q. et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci. Adv. 6, eaaw6071 (2020).
Tangpasuthadol, V., Pongchaisirikul, N. & Hoven, V. P. Surface modification of chitosan films. Eff. Hydrophobicity Protein Adsorpt. Carbohydr. Res 338, 937–942 (2003).
Hong, H. et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 232, 119679 (2020).
Ebhodaghe, S. O. Natural polymeric scaffolds for tissue engineering applications. J. Biomater. Sci. Polym. Ed. 32, 2144–2194 (2021).
Lewis, J. S. et al. Combinatorial delivery of immunosuppressive factors to dendritic cells using dual-sized microspheres. J. Mater. Chem. B 2, 2562–2574 (2014).
Firkowska-Boden, I., Zhang, X. & Jandt, K. D. Controlling protein adsorption through nanostructured polymeric surfaces. Adv. Healthc. Mater. 7, https://doi.org/10.1002/adhm.201700995 (2018).
Boddupalli, A., Zhu, L. & Bratlie, K. M. Methods for implant acceptance and wound healing: material selection and implant location modulate macrophage and fibroblast phenotypes. Adv. Health. Mater. 5, 2575–2594 (2016).
Horbett, T. A. Fibrinogen adsorption to biomaterials. J. Biomed. Mater. Res. A 106, 2777–2788 (2018).
Abdallah, M. N. et al. Biomaterial surface proteomic signature determines interaction with epithelial cells. Acta Biomater. 54, 150–163 (2017).
Babensee, J. E. Interaction of dendritic cells with biomaterials. Semin Immunol. 20, 101–108 (2008).
Gantner, B. N., Simmons, R. M., Canavera, S. J., Akira, S. & Underhill, D. M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med 197, 1107–1117 (2003).
Brown, G. D. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 6, 33–43 (2006).
Brand, U. et al. Influence of extracellular matrix proteins on the development of cultured human dendritic cells. Eur. J. Immunol. 28, 1673–1680 (1998).
Tsai, A. T. et al. The role of osteopontin in foreign body giant cell formation. Biomaterials 26, 5835–5843 (2005).
McNally, A. K., Jones, J. A., Macewan, S. R., Colton, E. & Anderson, J. M. Vitronectin is a critical protein adhesion substrate for IL-4-induced foreign body giant cell formation. J. Biomed. Mater. Res A 86, 535–543 (2008).
Keselowsky, B. G. et al. Role of plasma fibronectin in the foreign body response to biomaterials. Biomaterials 28, 3626–3631 (2007).
Escalante, T. et al. Wound exudate as a proteomic window to reveal different mechanisms of tissue damage by snake venom toxins. J. Proteome Res. 8, 5120–5131 (2009).
Swartzlander, M. D. et al. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials 41, 26–36 (2015).
Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12, 697–715 (1996).
Ryu, J. J., Park, K., Kim, H. S., Jeong, C. M. & Huh, J. B. Effects of anodized titanium with Arg-Gly-Asp (RGD) peptide immobilized via chemical grafting or physical adsorption on bone cell adhesion and differentiation. Int J. Oral. Maxillofac. Implants 28, 963–972 (2013).
Pierschbacher, M. D. & Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309, 30–33 (1984).
Hotaling, N. A. Elucidation and optimization of molecular factors for dendritic cell responses to surface presented glycans. Georgia Institute of Technology (2013).
Aukhil, I. Biology of wound healing. Periodontol 2000 22, 44–50 (2000).
Love, R. J. & Jones, K. S. The recognition of biomaterials: pattern recognition of medical polymers and their adsorbed biomolecules. J. Biomed. Mater. Res A 101, 2740–2752 (2013).
Rowley, A. T., Nagalla, R. R., Wang, S. W. & Liu, W. F. Extracellular matrix-based strategies for immunomodulatory biomaterials engineering. Adv. Health. Mater. 8, e1801578 (2019).
Jang, Y. H. et al. Molecular-level interactions between engineered materials and cells. Int. J. Mol. Sci. 20, https://doi.org/10.3390/ijms20174142 (2019).
Acharya, A. P. et al. The modulation of dendritic cell integrin binding and activation by RGD-peptide density gradient substrates. Biomaterials 31, 7444–7454 (2010).
McKiel, L. A. & Fitzpatrick, L. E. Toll-like receptor 2-dependent NF-κB/AP-1 activation by damage-associated molecular patterns adsorbed on polymeric surfaces. ACS Biomater. Sci. Eng. 4, 3792–3801 (2018).
Bianchi, M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 (2007).
Tancharoen, S. et al. HMGB1 promotes intraoral palatal wound healing through RAGE-dependent mechanisms. Int. J. Mol. Sci. 17, https://doi.org/10.3390/ijms17111961 (2016).
Ranathunga, D. T. S., Arteaga, A., Biguetti, C. C., Rodrigues, D. C. & Nielsen, S. O. Molecular-level understanding of the influence of ions and water on HMGB1 adsorption induced by surface hydroxylation of titanium implants. Langmuir 37, 10100–10114 (2021).
Biguetti, C. C. et al. HGMB1 and RAGE as essential components of Ti Osseointegration process in mice. Front Immunol. 10, 709 (2019).
Bennewitz, N. L. & Babensee, J. E. The effect of the physical form of poly(lactic-co-glycolic acid) carriers on the humoral immune response to co-delivered antigen. Biomaterials 26, 2991–2999 (2005).
Bethke, K. et al. Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: superiority of HSP60. J. Immunol. 169, 6141–6148 (2002).
Kato, S., Akagi, T., Sugimura, K., Kishida, A. & Akashi, M. Evaluation of biological responses to polymeric biomaterials by RT-PCR analysis III: study of HSP 70, 90 and 47 mRNA expression. Biomaterials 19, 821–827 (1998).
Zhou, J., An, H., Xu, H., Liu, S. & Cao, X. Heat shock up-regulates expression of Toll-like receptor-2 and Toll-like receptor-4 in human monocytes via p38 kinase signal pathway. Immunology 114, 522–530 (2005).
Wilson, C. J., Clegg, R. E., Leavesley, D. I. & Pearcy, M. J. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 11, 1–18 (2005).
Hirata, T. et al. Effect of local chain dynamics on a bioinert interface. Langmuir 31, 3661–3667 (2015).
Wu, J., Lin, W., Wang, Z., Chen, S. & Chang, Y. Investigation of the hydration of nonfouling material poly(sulfobetaine methacrylate) by low-field nuclear magnetic resonance. Langmuir 28, 7436–7441 (2012).
Hašek, J. Poly (ethylene glycol) interactions with proteins. Z. Kristallogr. Suppl 23, 613–618 (2006).
Kang, B., Opatz, T., Landfester, K. & Wurm, F. R. Carbohydrate nanocarriers in biomedical applications: functionalization and construction. Chem. Soc. Rev. 44, 8301–8325 (2015).
Patwa, T. H., Zhao, J., Anderson, M. A., Simeone, D. M. & Lubman, D. M. Screening of glycosylation patterns in serum using natural glycoprotein microarrays and multi-lectin fluorescence detection. Anal. Chem. 78, 6411–6421 (2006).
Keselowsky, B. G., Collard, D. M. & García, A. J. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res A 66, 247–259 (2003).
Shankar, S. P., Chen, I. I., Keselowsky, B. G., García, A. J. & Babensee, J. E. Profiles of carbohydrate ligands associated with adsorbed proteins on self-assembled monolayers of defined chemistries. J. Biomed. Mater. Res A 92, 1329–1342 (2010).
Shankar, S. P., Petrie, T. A., García, A. J. & Babensee, J. E. Dendritic cell responses to self-assembled monolayers of defined chemistries. J. Biomed. Mater. Res A 92, 1487–1499 (2010).
Chalabi, S. et al. Differential O-glycosylation of a conserved domain expressed in murine and human ZP3. Biochemistry 45, 637–647 (2006).
Cornelius, R. M., Shankar, S. P., Brash, J. L. & Babensee, J. E. Immunoblot analysis of proteins associated with self-assembled monolayer surfaces of defined chemistries. J. Biomed. Mater. Res. A 98, 7–18 (2011).
Kou, P. M. & Babensee, J. E. Validation of a high-throughput methodology to assess the effects of biomaterials on dendritic cell phenotype. Acta Biomater. 6, 2621–2630 (2010).
Kou, P. M. et al. Predicting biomaterial property-dendritic cell phenotype relationships from the multivariate analysis of responses to polymethacrylates. Biomaterials 33, 1699–1713 (2012).
Yang, J. et al. Polymer surface functionalities that control human embryoid body cell adhesion revealed by high throughput surface characterization of combinatorial material microarrays. Biomaterials 31, 8827–8838 (2010).
Garcia-Cordero, J. L., Nembrini, C., Stano, A., Hubbell, J. A. & Maerkl, S. J. A high-throughput nanoimmunoassay chip applied to large-scale vaccine adjuvant screening. Integr. Biol. 5, 650–658 (2013).
Sinha, N., Subedi, N. & Tel, J. Integrating immunology and microfluidics for single immune cell analysis. Front Immunol. 9, 2373 (2018).
Hotaling, N. A., Tang, L., Irvine, D. J. & Babensee, J. E. Biomaterial strategies for immunomodulation. Annu. Rev. Biomed. Eng. 17, 317–349 (2015).
Kapp, T. G., Rechenmacher, F., Sobahi, T. R. & Kessler, H. Integrin modulators: a patent review. Expert Opin. Ther. Pat. 23, 1273–1295 (2013).
Winograd-Katz, S. E., Fässler, R., Geiger, B. & Legate, K. R. The integrin adhesome: from genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 15, 273–288 (2014).
Schittenhelm, L., Hilkens, C. M. & Morrison, V. L. β(2) Integrins as regulators of dendritic cell, monocyte, and macrophage function. Front. Immunol. 8, 1866 (2017).
Benvenuti, F. The dendritic cell synapse: a life dedicated to T cell activation. Front. Immunol. 7, 70 (2016).
Ling, G. S. et al. Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nat. Commun. 5, 3039 (2014).
Rogers, T. H. & Babensee, J. E. The role of integrins in the recognition and response of dendritic cells to biomaterials. Biomaterials 32, 1270–1279 (2011).
Franz, S., Rammelt, S., Scharnweber, D. & Simon, J. C. Immune responses to implants—a review of the implications for the design of immunomodulatory. Biomater. Biomater. 32, 6692–6709 (2011).
de Fougerolles, A. R. et al. Global expression analysis of extracellular matrix-integrin interactions in monocytes. Immunity 13, 749–758 (2000).
van Vliet, S. J., den Dunnen, J., Gringhuis, S. I., Geijtenbeek, T. B. & van Kooyk, Y. Innate signaling and regulation of Dendritic cell immunity. Curr. Opin. Immunol. 19, 435–440 (2007).
Yoshida, M. & Babensee, J. E. Molecular aspects of microparticle phagocytosis by dendritic cells. J. Biomater. Sci. Polym. Ed. 17, 893–907 (2006).
Gay, N. J., Symmons, M. F., Gangloff, M. & Bryant, C. E. Assembly and localization of Toll-like receptor signalling complexes. Nat. Rev. Immunol. 14, 546–558 (2014).
Satoh, T. & Akira, S. Toll-like receptor signaling and its inducible proteins. Microbiol. Spectr. 4, (2016) https://doi.org/10.1128/microbiolspec.MCHD-0040-2016
Rogers, T. H. & Babensee, J. E. Altered adherent leukocyte profile on biomaterials in Toll-like receptor 4 deficient mice. Biomaterials 31, 594–601 (2010).
Fore, F., Indriputri, C., Mamutse, J. & Nugraha, J. TLR10 and its unique anti-inflammatory properties and potential use as a target in therapeutics. Immune Netw. 20, e21 (2020).
Balenga, N. A. & Balenga, N. A. Human TLR11 gene is repressed due to its probable interaction with profilin expressed in human. Med. Hypotheses 68, 456 (2007).
Celhar, T., Magalhães, R. & Fairhurst, A. M. TLR7 and TLR9 in SLE: when sensing self goes wrong. Immunol. Res. 53, 58–77 (2012).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Lin, S. C., Lo, Y. C. & Wu, H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 465, 885–890 (2010).
Pearl, J. I. et al. Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials 32, 5535–5542 (2011).
Ajibade, A. A., Wang, H. Y. & Wang, R. F. Cell type-specific function of TAK1 in innate immune signaling. Trends Immunol. 34, 307–316 (2013).
Chen, Z. J. Ubiquitination in signaling to and activation of IKK. Immunol. Rev. 246, 95–106 (2012).
May, M. J. & Ghosh, S. Signal transduction through NF-kappa B. Immunol. Today 19, 80–88 (1998).
Mitchell, S., Vargas, J. & Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 8, 227–241 (2016).
O’Neill, L. A. & Bowie, A. G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7, 353–364 (2007).
Flo, T. H. et al. Involvement of toll-like receptor (TLR) 2 and TLR4 in cell activation by mannuronic acid polymers. J. Biol. Chem. 277, 35489–35495 (2002).
Maitra, R., Clement, C. C., Crisi, G. M., Cobelli, N. & Santambrogio, L. Immunogenecity of modified alkane polymers is mediated through TLR1/2 activation. PLoS One 3, e2438 (2008).
Chen, H. et al. The promotion of type 1T helper cell responses to cationic polymers in vivo via toll-like receptor-4 mediated IL-12 secretion. Biomaterials 31, 8172–8180 (2010).
Amer, L. D. et al. Inflammation via myeloid differentiation primary response gene 88 signaling mediates the fibrotic response to implantable synthetic poly(ethylene glycol) hydrogels. Acta Biomater. 100, 105–117 (2019).
Wang, L. et al. Manipulation of macrophage polarization by peptide-coated gold nanoparticles and its protective effects on acute lung injury. J. Nanobiotechnology 18, 38 (2020).
Padovan, E., Landmann, R. M. & De Libero, G. How pattern recognition receptor triggering influences T cell responses: a new look into the system. Trends Immunol. 28, 308–314 (2007).
Mazgaeen, L. & Gurung, P. Recent advances in lipopolysaccharide recognition systems. Int. J. Mol. Sci. 21, (2020) https://doi.org/10.3390/ijms21020379
Andersson, U. & Tracey, K. J. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 29, 139–162 (2011).
Jiang, D., Liang, J. & Noble, P. W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 91, 221–264 (2011).
Oliveira, M. I. et al. Adsorbed Fibrinogen stimulates TLR-4 on monocytes and induces BMP-2 expression. Acta Biomater. 49, 296–305 (2017).
Auquit-Auckbur, I. et al. Role of toll-like receptor 4 in the inflammation reaction surrounding silicone prosthesis. Acta Biomater. 7, 2047–2052 (2011).
Uto, T. et al. The induction of innate and adaptive immunity by biodegradable poly(γ-glutamic acid) nanoparticles via a TLR4 and MyD88 signaling pathway. Biomaterials 32, 5206–5212 (2011).
Bojarová, P. & Křen, V. Sugared biomaterial binding lectins: achievements and perspectives. Biomater. Sci. 4, 1142–1160 (2016).
Lepenies, B., Lee, J. & Sonkaria, S. Targeting C-type lectin receptors with multivalent carbohydrate ligands. Adv. Drug Deliv. Rev. 65, 1271–1281 (2013).
van Kooyk, Y. & Rabinovich, G. A. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 9, 593–601 (2008).
van Kooyk, Y. C-type lectins on dendritic cells: key modulators for the induction of immune responses. Biochem. Soc. Trans. 36, 1478–1481 (2008).
García-Vallejo, J. J. et al. Multivalent glycopeptide dendrimers for the targeted delivery of antigens to dendritic cells. Mol. Immunol. 53, 387–397 (2013).
Bonifaz, L. et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+T cell tolerance. J. Exp. Med. 196, 1627–1638 (2002).
Chen, Y., De Koker, S. & De Geest, B. G. Engineering strategies for lymph node targeted immune activation. Acc. Chem. Res. 53, 2055–2067 (2020).
Hotaling, N. A., Cummings, R. D., Ratner, D. M. & Babensee, J. E. Molecular factors in dendritic cell responses to adsorbed glycoconjugates. Biomaterials 35, 5862–5874 (2014).
Hotaling, N. A., Ratner, D. M., Cummings, R. D. & Babensee, J. E. Presentation modality of Glycoconjugates modulates dendritic cell phenotype. Biomater. Sci. 2, 1426–1439 (2014).
Geijtenbeek, T. B., van Vliet, S. J., Engering, A. T., Hart, B. A. & van Kooyk, Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu. Rev. Immunol. 22, 33–54 (2004).
Dambuza, I. M. & Brown, G. D. C-type lectins in immunity: recent developments. Curr. Opin. Immunol. 32, 21–27 (2015).
Hatscher, L., Amon, L., Heger, L. & Dudziak, D. Inflammasomes in dendritic cells: Friend or foe? Immunol. Lett. 234, 16–32 (2021).
Lamkanfi, M. & Dixit, V. M. Modulation of inflammasome pathways by bacterial and viral pathogens. J. Immunol. 187, 597–602 (2011).
He, Y., Hara, H. & Núñez, G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 41, 1012–1021 (2016).
Seydoux, E. et al. Effective combination adjuvants engage both TLR and inflammasome pathways to promote potent adaptive immune responses. J. Immunol., https://doi.org/10.4049/jimmunol.1701604
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009).
Zhivaki, D. et al. Inflammasomes within hyperactive murine dendritic cells stimulate long-lived T cell-mediated anti-tumor immunity. Cell Rep. 33, 108381 (2020).
Shaw, P. J., Mcdermott, M. F. & Kanneganti, T. D. Inflammasomes and autoimmunity. Trends Mol. Med. 17, 57–64 (2011).
Li, W. A., Lu, B. Y., Luo, G., Choi, Y. & Mooney, D. J. The effect of surface modification of mesoporous silica micro-rod scaffold on immune cell activation and infiltration. Biomaterials 83, 249–256 (2016).
Sharp, F. A. et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc. Natl Acad. Sci. USA 106, 870–875 (2009).
Si, Y. et al. MyD88 in antigen-presenting cells is not required for CD4+ T-cell responses during peptide nanofiber vaccination. Med. Chem. Commun. 9, 138–148 (2018).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Saikat, M. et al. Immunomodulation of the NLRP3 inflammasome through structure-based activator design and functional regulation via lysosomal rupture. ACS Cent. Sci., (2018) https://doi.org/10.1021/acscentsci.8b0021.
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462 (2014).
Malinarich, F. et al. High mitochondrial respiration and glycolytic capacity represent a metabolic phenotype of human tolerogenic dendritic cells. J. Immunol. 194, 5174–5186 (2015).
Kim, J., Kundu, M., Viollet, B. & Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 (2011).
Hulea, L., Markovic, Z., Topisirovic, I., Simmet, T. & Trajkovic, V. Biomedical potential of mTOR modulation by nanoparticles. Trends Biotechnol. 34, 349–353 (2016).
Sukhbaatar, N., Hengstschläger, M. & Weichhart, T. mTOR-mediated regulation of dendritic cell differentiation and function. Trends Immunol. 37, 778–789 (2016).
Feng, Y., Yao, Z. & Klionsky, D. J. How to control self-digestion: transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol. 25, 354–363 (2015).
Volarevic, V. et al. Large graphene quantum dots alleviate immune-mediated liver damage. ACS Nano 8, 12098–12109 (2014).
Qin, Y. et al. Graphene quantum dots induce apoptosis, autophagy, and inflammatory response via p38 mitogen-activated protein kinase and nuclear factor-κB mediated signaling pathways in activated THP-1 macrophages. Toxicology 327, 62–76 (2015).
Chang, C. P., Su, Y. C., Hu, C. W. & Lei, H. Y. TLR2-dependent selective autophagy regulates NF-κB lysosomal degradation in hepatoma-derived M2 macrophage differentiation. Cell Death Differ. 20, 515–523 (2013).
Said, A. Bock, S. Lajqi, T. Müller, G. Weindl, G. Chloroquine promotes IL-17 production by CD4+T cells via p38-dependent IL-23 release by monocyte-derived Langerhans-like cells. J. Immunol. 193, 6135–6145 (2014) https://doi.org/10.4049/jimmunol.1303276.
Acknowledgements
This study was supported by the Key Research and Development Program of Science and Technology Department of Zhejiang Province (No. 2019C03081).
Author information
Authors and Affiliations
Contributions
F.H., S.W., and Y.C. conceived of the presented idea. S.W., Y.C., Z.L., and J.L discussed the review contents. S.W., Y.C., Z.L., and J.L wrote the manuscript, J.H, Q.C. and F.H. edited the manuscript. S.W. and Z.L. designed the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, S., Chen, Y., Ling, Z. et al. The role of dendritic cells in the immunomodulation to implanted biomaterials. Int J Oral Sci 14, 52 (2022). https://doi.org/10.1038/s41368-022-00203-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41368-022-00203-2
This article is cited by
-
Role of dendritic cells in MYD88-mediated immune recognition and osteoinduction initiated by the implantation of biomaterials
International Journal of Oral Science (2023)
-
The HDAC inhibitor zabadinostat is a systemic regulator of adaptive immunity
Communications Biology (2023)