Abstract
Background/Objectives
Weight gain is a barrier to smoking cessation. Previous interventions targeting weight gain while quitting smoking have largely been unsuccessful. The current study aimed to assess the efficacy of weight stability and weight loss interventions compared to a low-intensity, self-guided bibliotherapy weight management group.
Subjects/Methods
A randomized controlled trial with 12-month follow-up from 2018 to 2022 was conducted with participants (N = 305) who reported smoking at least five cigarettes per day for the last year and interest in quitting initially recruited from the Memphis, TN, USA area. Recruitment was expanded nationally with the onset of the COVID-19 pandemic. Subsequently, 276 completed 12-month follow-up.
Interventions/Methods
The Bibliotherapy group was provided a weight management book. Both the Stability and Loss groups met via telephone for eight weeks to learn strategies for maintaining/losing weight, respectively. All three groups then received the same six-week smoking cessation intervention, with six months of varenicline provided.
Results
Individuals in the Loss group lost more weight (−2.01 kg, SE = 1.58) than individuals in the Bibliotherapy group (+1.08 kg, SE = 1.49, p = 0.0004), while the Stability group (−0.30 kg, SE = 1.56) was not significantly different from the Bibliotherapy group (p = 0.17). Those in the Stability group did not gain a significant amount of weight. Participants in the Loss group did not gain back all weight lost after smoking cessation and ended the study approximately 2.01 kg lower than baseline. The Bibliotherapy group did not gain the amount of weight expected after cessation. There were no significant differences between groups related to self-reported smoking cessation at each time point except at eight-month follow-up (p = 0.005).
Conclusions and relevance
Results indicated the Stability and the Loss interventions were effective for preventing post-smoking cessation weight gain, with the Loss group having the benefit of sustained weight loss. These interventions may be helpful to implement to combat weight gain and potentially facilitate smoking cessation.
Trial Registration
The trial is registered on clinicaltrials.gov (NCT03156660).

Similar content being viewed by others
Introduction
Smoking remains the leading cause of preventable death in the United States [1]. While approximately 68.0% of individuals who smoke report they want to quit, only 7.4% actually achieve cessation with each quit attempt [2]. Smoking cessation is associated with weight gain due to decreased metabolic rate and increased energy intake [3], with individuals gaining approximately 1.1 kg, 2.3 kg, 2.9 kg, 4.2 kg and 4.7 kg at 1, 2, 3, 6, and 12 months after quitting smoking, respectively [4], with greater weight gains seen among those with a body mass index (BMI) in healthy and overweight ranges [5]. Yet, smoking cessation reduces a person’s risk for cardiovascular diseases [6, 7], even if they gain weight. However, weight gain is also a commonly cited reason for either not trying to quit smoking or smoking relapse [8,9,10], particularly among women [3, 11]. Given the importance of weight gain in relation to smoking cessation, it may be prudent to tackle both issues to effectively promote sustained smoking abstinence.
Numerous studies have evaluated interventions that target weight gain among individuals who attempt smoking cessation. According to one systematic review and meta-analysis, individuals who received a variety of combined smoking and weight control treatments simultaneously were more likely to be abstinent from smoking and see reductions in weight, although effect sizes related to weight loss were small, and effects were no longer significant after six months [12]. A more recent review focused on post-cessation weight management studies [13] concluded, “there is no intervention for which there is moderate certainty of a clinically useful effect on long‐term weight gain,” although there was some indication that exercise interventions may reduce weight gain. Thus, there is a need to identify and test interventions that may preclude post-cessation weight gain and promote long-term cessation.
Teaching weight maintenance skills prior to engaging in weight loss efforts has shown promising results for reducing the amount of weight gained back over time [14, 15]. Specifically, Wing and colleagues [14] introduced a “small changes” intervention where participants made approximately 100-calorie changes by reducing dietary intake and increased activity, which was associated with reduced incidence of obesity [14]. While these trials were not conducted in the context of smoking cessation, teaching individuals to be weight “stable” may effectively translate to the post-cessation period where weight gain is typical.
Another promising area of research has been interventions that promote weight loss, such as the Look AHEAD Intensive Lifestyle Intervention [16]. This intervention has led to long-term weight reductions and may be helpful with facilitating weight loss prior to a cessation attempt to compensate for post-cessation weight gain. However, this intervention has not yet been tested among individuals looking to quit smoking.
The study goal was to conduct a randomized controlled trial to test the efficacy of two interventions that aimed to reduce post-cessation weight gain. We tested a weight stability intervention and a weight loss intervention compared to a low-intensity, self-guided bibliotherapy weight management group. We expected participants randomized to either the Loss or Stability groups would experience significantly less post-cessation weight gain at 12 months than the Bibliotherapy group. We also expected the Loss and Stability groups would not significantly differ in their post-cessation weight loss, and all groups would experience similar smoking cessation rates.
Materials/subjects and methods
Participants and procedures
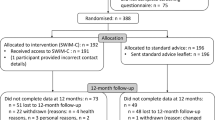
All procedures were approved by the University of Tennessee Health Science Center Institutional Review Board, and the trial was prospectively registered at clinicaltrials.gov (NCT03156660). Figure 1 displays the study diagram, including the number of participants screened and assessed at each follow-up visit. Participants were 305 individuals ages 18 and older recruited initially from Memphis, Tennessee and surrounding areas (within 50 miles). During the COVID-19 pandemic, recruitment was expanded nationally. Participants were recruited from 2018 to 2021, and final data collection visits occurred in 2022. Potential participants completed screening questions to determine eligibility, including if they had smoked at least five cigarettes per day for the last year, were interested in quitting smoking in the next 30 days, and had access to a telephone and email for intervention delivery. Power analyses and full inclusion/exclusion criteria are described elsewhere [17]. Initial power analysis indicated recruitment of about 400 individuals. However, initial recruitment was slow and thus intervention groups were started once some recruitment waves were approximately 75% full, which prevented participants from waiting more than a few months to start intervention. Once a group started, it was closed to further accrual. Additionally, due to the COVID-19 pandemic, recruitment was paused for about five months. Thus, we were unable to recruit all 400 participants within the funding period.
Phone screening procedures and descriptions of subsequent visits are also described in detail elsewhere [17]. Briefly, potential participants who passed the phone screening were scheduled for a screening visit, where staff obtained informed consent. Participants completed self-report and physical measures. Participants were then assigned behavioral run-in tasks, including a three-day diet and exercise journal and obtained physician clearance to participate. Participants deemed eligible at the screening visit then were scheduled for a baseline visit to complete further questionnaires, have weight measured, and submit their behavioral run-in tasks.
Participants also had a randomization visit where they were informed of their random assignment to one of three intervention groups by study staff. An adaptive randomization approach was used accounting for constraints such as participant availability and different group sizes while balancing the equality between the groups regarding demographic and clinical characteristics. The goal initially was to use a 2:2:1 randomization strategy, with fewer in the Bibliotherapy condition. However, due to slow initial recruitment and the concern about having groups that were too small in the Bibliotherapy condition, we modified the randomization scheme to be even randomization across the three conditions, with Data Safety and Monitoring Board guidance. Randomization procedures were conducted by the statistician (ZB).
Intervention procedures are described in detail elsewhere [17]. Briefly, the first group was the weight stability group (“Stability”), who received a Fitbit activity tracker and a step goal (i.e., increase by 2000–3000 steps per day over their baseline levels). Fitbits distributed changed from Alta to Inspire during the trial due to discontinuation of the Alta, although both had similar features. The first eight weeks of this intervention focused on making small 100-calorie changes to maintain a stable weight. Participants were encouraged to keep their weight stable during this period using their personalized, color-coded weight trajectory graph as feedback: red (i.e., weight ranges encouraging more than one small change), yellow (i.e., weight ranges suggesting one small change), and green (i.e., optimal weight of ±3 lbs. of baseline weight) zones. Stability participants were mailed small green prizes each week, such as a pen or gum, if they stayed in the green zone.
Second, the weight loss group (“Loss”) received eight weeks of meal replacements for two meals per day (i.e., Slim Fast powder, oatmeal packets, and snack bars) to assist in reaching their calorie and fat goals based on baseline weight. Loss participants were also given a blender bottle to help mix Slim Fast shakes, measuring cups and spoons to measure portion sizes, and a Fitbit Alta or Inspire to track progress toward their physical activity goals (i.e., at least 175 min of moderate intensity exercise per week or 10,000 steps per day).
Third, the self-guided bibliotherapy group (“Bibliotherapy”) received “The Eating Well Diet®” book [18] about weight management and asked to use this book independently for the first eight weeks. This group was not contacted during the weight management period unless they called study staff with questions related to their participation.
All participants received an electronic BodyTrace scale and were recommended to weigh every day. Study staff and participants were able to access their measurements using a scale-specific link to monitor weighing frequency and weight trajectory over time, and interventionists provided emailed feedback to participants in Stability and Loss groups on the same schedule as the group sessions (i.e., weekly and then monthly).
Participants completed follow-up visits at 2, 4, 8, and 12 months after baseline, where they completed self-report questionnaires, cotinine tests if they reported smoking abstinence, and physical measurements (i.e., weight). They were given $30 Amazon gift cards for completing the 4-month follow-up visit, and $35 Amazon gift cards for the 8-month and 12-month follow-up visits. All visits were in-person until the COVID-19 pandemic onset, when visits were conducted remotely beginning in March 2020. Staff facilitating follow-up visits were blinded to participant group assignments.
The first eight weeks of each group were focused on the assigned weight intervention. The Stability and Loss groups met for one hour each week via telephone using HIPAA-compliant Zoom and were recorded for treatment fidelity monitoring by a co-investigator (KD). After the weight intervention, all groups received a six-week behavioral smoking cessation intervention (also recorded for treatment fidelity monitoring) plus six months of varenicline pharmacotherapy. The smoking cessation intervention consisted of six 60-min group sessions and one individual session focused on the quitting process and relapse prevention. Participants were encouraged to set a quit date after the second smoking cessation session. All group sessions followed materials emailed to participants. Motivational interviewing was used to facilitate discussions and practice behavioral skills, and groups were led by trained interventionists, with continuity of the same interventionist for the Stability and Loss groups. Five monthly booster sessions were conducted for the Stability and Loss groups after the smoking cessation intervention completion, to bolster weight management skills.
Participants also completed five medication calls to measure medication adherence, assess for side effects, and troubleshoot issues with taking the medication (e.g., reducing dose if side effects were significant/intolerable). Adverse events were reported by staff and monitored by a study physician. Participants who did not take the full dose each day (two 1 milligram tablets after gradually increasing their dose from the starter pack) and therefore had excess pills were not mailed each box but were sent another box once they required additional pills to prevent participants from taking the medication for longer compared to other participants. Of note, Chantix™ was recalled in July 2021, which was the varenicline source for most of the study. Participants who had been administered Chantix™ were called and instructed to discard their Chantix™. Generic varenicline was imported from Canada and sent to participants in the final wave. All varenicline was recalled in September 2021, and participants were alerted to the recall (with some participants in final study wave discontinuing their last box).
Measures
Demographics
Demographic characteristics such as age, race, ethnicity, income, marital status, and sex were measured at the screening visit.
Point prevalence
At every data collection visit, participants were asked if they had smoked a cigarette, even just a puff, in the past seven days. Answer options were dichotomous.
Fagerstrom test of nicotine dependence (FTND)
Nicotine dependence was measured using the FTND at the randomization and follow-up visits where participants reported smoking in the past 24 h. This is a standardized six-item measure, with higher scores indicating higher nicotine dependence [19]. Items included “Do you find it difficult to refrain from smoking in places where it is forbidden, e.g., in a church, at the library, cinema, etc.?” and “Which cigarettes would you hate most to give up?”
Biochemical verification of smoking abstention
At each follow-up visit starting at two-month follow-up, participants who reported not smoking in the past 24 h were asked to take a cotinine test to biochemically verify their smoking abstinence. NicAlert™ was used prior to the COVID-19 pandemic [17]. At in-person visits, participants were asked to spit into a collection container, and a NicAlert™ test strip was used by staff to test cotinine levels. After the COVID-19 pandemic started, participants were mailed the iScreenTM cotinine test, where they swabbed their mouth with a sponge connected to a cotinine test for three minutes and sent a photo of the result to staff. This procedure started four months after the pandemic started, so no biochemical verification occurred for four-months while the study team adjusted procedures. Additionally, prior to the COVID-19 pandemic, a SmokerlyzerTM test was administered to test exhaled carbon monoxide.
Weight
Weight was measured at each in-person visit using a calibrated scale prior to COVID-19, and by BodyTrace scale for each visit after the pandemic started. Two measurements were taken for each visit. If the measurements were more than 0.2 kilograms different, a third weight was taken. Weight measurements were then averaged for a final value. The BodyTrace scale has been shown to be an accurate measure of weight [20].
Data analysis
All analyses were performed with SAS/STATv15.2. Descriptive statistics were generated for continuous and categorical variables, respectively, overall and by condition. Frequencies and proportions were used to describe self-reported and biochemically verified abstinence pattern by condition across follow-up time points. Means and standard deviations were calculated to describe unadjusted weight pattern by condition across follow-up time points. An intention-to-treat analysis using fully conditional specification (FCS) model based multiple imputations of weight data (25 iterations) as well as a completers analysis were conducted. The model assumed data was missing at random (MAR). The primary outcome was weight change from baseline to 12-month follow-up and was initially modeled using the analysis of covariance (ANCOVA) general linear model. This difference was modeled as a function of condition, with the Bibliotherapy condition as a comparison condition, while adjusting for baseline weight. Another model adjusting for baseline weight, age, sex, BMI, race, ethnicity, marital status, education, income, screening visit FTND score, and screening visit average cigarettes per day was also conducted. Bonferroni adjusted p-values were reported to account for multiple comparisons between conditions. An additional ANCOVA model was constructed, adjusting for the same covariates, as well as point prevalence and an interaction term between intervention group and point prevalence to determine if there was a differential effect between intervention group and change in weight by smoking status. Alpha level was set at 0.05 within the context of all available evidence such as intervention effects, variability, and confidence limits.
Results
Participant characteristics
Table 1 displays participant characteristics overall and within each group. Overall, the sample was mostly women (67.9%), and most had a college education (70.2%) and were not married (57.1%). Additionally, 52.1% identified as White, 43.3% identified as Black, and 86.9% had overweight or obesity. Average age was 54.3 years (standard deviation [SD] = 11.6). Overall, 10.8% of participants had missing outcome data at 12 months with no differential attrition between conditions (p = 0.41). Individuals who identified as Black (p = 0.03) and participants with lower incomes (p = 0.002) had significantly more missing outcome data at 12 months. This difference was accounted for in our models. Average weight and smoking status across groups at each time point is displayed in Figs. 2 and 3.
Bar graphs showing proportions of self-reported and biochemically-verified abstinence at 2 month, 4 month, 8 month, and 12 month follow-up visits for the three conditions: (a) Stability, (b) Loss, and (c) Bibliotherapy. At each follow-up visit, participants who reported not smoking in the past 24 h were asked to take a cotinine test (pre-COVID-19 pandemic: NicAlert™; during the COVID-19 pandemic: iScreen™) to biochemically verify their smoking abstinence. a the abstinence at the 2 month follow-up visit, b at the 4 month follow-up visit, c at the 8 month follow-up visit, and d at the 12 month follow-up visit. Biochemically verified smoking abstinence rates were largely similar to the self-reported results.
Treatment fidelity
Mean treatment fidelity score for content discussion was 98.18 out of 100 (SD = 7.02). Mean Global Rating of Motivational Interviewing was 4.08 out of 5 (SD = 0.76).
Smoking
There were no significant differences between groups related to self-reported quitting smoking at each time point except at 8-month follow-up (p = 0.005). The Stability group had the greatest proportion of participants smoking at 8-months (61.7%), as several individuals appear to have had a lapse or relapse (smoking rates increased from 52.9% at 4-month follow-up). The Bibliotherapy group had the lowest proportion of participants smoking at 37.0% at 8-months. Biochemically verified smoking abstinence rates were largely similar to the self-reported results (Fig. 2, Supplementary Table 1), with quit status again only being significantly different at the 8-month time point (p = 0.003). Among participants who received a NicAlert test, 82% were confirmed abstinent and among those with iScreen tests, 94% were confirmed abstinent. At 12-month follow-up, almost half reported quitting smoking (41.1%-51.3%).
Changes in weight
Results from the intent-to-treat analyses indicated the Stability condition had a mean weight loss of 0.24 kg (SD = 0.25) from baseline to 12 months. The Loss condition had a mean weight loss of 1.11 kg (SD = 0.20), and the Bibliotherapy group had a mean weight gain of 1.25 kg (SD = 0.27). Weight change for the Loss condition was significantly different from the Bibliotherapy condition (p = 0.009); however, weight change for the Stability condition was not significantly different from the Bibliotherapy condition (p = 0.30). Results were similar in the completers analysis (Fig. 3, Supplementary Table 2). Specifically, the Stability condition had a mean weight loss of 0.30 kg (SE = 0.62), the Loss condition had a mean weight loss of 1.29 kg (SE = 0.60), and the Bibliotherapy group had a mean weight gain of 1.32 kg (SE = 0.68). Again, weight change for the Loss condition was significantly different from the Bibliotherapy condition (p = 0.01), and weight change for the Stability condition was not significantly different from the Bibliotherapy condition (p = 0.20).
Results from ANCOVA model controlling for baseline weight, age, sex, BMI, race, ethnicity, marital status, education, income, FTND score, and average cigarettes per day (Table 2) found the Loss group lost an average of 2.01 kg (SE = 1.58), which was significantly different from the Bibliotherapy group (B = −3.09, SE = 0.95, p = 0.004), who gained an average of 1.08 kg (SE = 1.49). The Stability group lost an average of 0.30 kg (SE = 1.56), which was not significantly different from the Bibliotherapy group (B = −1.38, SE = 0.96, p = 0.46). Additionally, there were no significant differences in weight based on the measured demographic characteristics (p > 0.05).
In the ANCOVA model adjusting for similar covariates, point prevalence, and the interaction between intervention group and point prevalence (Table 2), we found the interaction term was not statistically significant (p = 0.07). However, it is worth noting some differential weight change patterns within intervention groups by smoking status. Participants who quit smoking in the Stability intervention gained 2.04 kg (SE = 1.70), while participants who were not abstinent at the 12-month follow-up visit in the Stability intervention lost 2.57 kg (SE = 1.65). Participants who quit smoking in the Loss intervention lost 2.18 kg (SE = 1.69), and participants who did not quit smoking at 12 months lost 2.81 kg (SE = 1.66). Those who quit smoking in the Bibliotherapy condition gained 1.55 kg (SE = 1.55), but those who smoked at the 12-month visit lost 0.17 kg (SE = 1.71). A post hoc comparison between Stability and Loss interventions for participants who were abstinent from smoking revealed a significant difference of 4.2 kg (SE = 1.48, adjusted p = 0.02).
Discussion
The current study found individuals in a weight loss intervention lost significantly more weight than the Bibliotherapy group, while the weight change in the weight stability intervention was not significantly different from the Bibliotherapy condition. It was notable the participants in the Loss group did not gain back all weight that they lost after smoking cessation, which was expected, and ended the study approximately 2.01 kg lower than baseline. This is particularly noteworthy given that individuals typically gain about 4.7 kg in the year they quit smoking [4], and sustaining weight loss in the year after quitting smoking indicates this intervention may be very effective. These results indicate this structured behavioral weight loss intervention may be the best choice for a weight management intervention to combine with smoking cessation treatment.
Additionally, participants in the Bibliotherapy arm did not behave as expected. They were expected to gain an average of five kg after smoking cessation, similar to what has been reported in previous studies related to weight gain after cessation [4, 21]. It remains unclear as to why this group was different than other samples; it may be that the participants in this “control group” were particularly motivated to achieve weight loss and smoking cessation goals since they knew the study design was to spend the first eight weeks focusing on weight before the smoking cessation intervention. It is also possible the minimal self-guided intervention was more potent than anticipated. Lastly, it could be, once individuals were involved in the smoking cessation group, they felt they had a supportive environment that helped them to cope with smoking cessation difficulties rather than using food to cope. More research is needed to better understand if this Bibliotherapy intervention was effective in preventing excessive weight gain or if these participants were unique.
Additionally, while the Stability group was not significantly different from the Bibliotherapy group, this is potentially attributable to the Bibliotherapy group behaving inconsistently with expectations [12]. The Stability group achieved what was expected; this group did not gain weight (mean weight loss of 0.24 kg). Taken together, the Loss and Stability groups seem to have been effective in preventing weight gain after smoking cessation, with the Loss group having increased benefit of small, sustained weight loss even after 12-month follow-up. Future research may benefit from assessing other considerations such as cost-effectiveness to better determine which intervention may be most appropriate for implementation.
It is also notable there were no differences in weight change across demographic groups, which may indicate these interventions are helpful across diverse populations. More research is needed to replicate this finding and determine if this is due to the intervention itself or other factors, such as staff diversity that may have contributed to a more inclusive environment.
A large proportion of participants successfully quit smoking at 12-month follow-up, with almost half of each group reporting abstinence [22]. This finding is consistent with other studies using varenicline for smoking cessation, with a recent review reporting studies saw continued abstinence rates ranging from approximately 29% to about 65%. There was also high agreement between self-reported and biochemically verified smoking abstinence. Past studies have shown significant disagreement between the two, with self-report often showing much higher abstinence rates [23, 24]. It may be the case that rapport with research staff encouraged honest reporting of smoking status. More research is needed to identify factors that may have contributed to this more accurate self-reporting, as this could be beneficial to implement in future studies to improve outcome data reliability.
Lastly, there were some differences in the amount of weight gained or lost based on smoking status at 12-month follow-up, with those who did not quit smoking having higher weight loss compared to those who did quit. Although these values were not statistically significant, they are an important consideration for future research given that those who successfully quit smoking did see less weight loss or more weight gained, and this has been a deterrent to maintaining a quit in previous studies [3, 8,9,10,11]. It will be important for future studies implementing these interventions to continue evaluating differences in weight change by smoking status, as individuals may be at risk for relapse should their weight significantly increase compared to their smoking counterparts.
Strengths and limitations
Despite the study strengths, including the novelty of the interventions being tested, the remote delivery of the intervention (which may facilitate dissemination), the diverse sample, and the high fidelity to the intervention protocol, there are notable limitations. ChantixTM was recalled during the study, which may have impacted medication adherence towards the end of the study. Participants who were administered generic varenicline after being told to discard their ChantixTM for safety concerns may have been concerned about taking more varenicline and may have been less adherent, potentially impacting their cessation outcome. Another limitation is that few individuals were recruited who identified with racial identities other than White or Black; it will be important to replicate these findings in other racial groups (e.g., Asian or Native American). Finally, several protocol changes were required due to the COVID-19 pandemic; however, the successful implementation of these modifications demonstrates the ability to conduct this study remotely, allowing national access. The COVID-19 pandemic also slowed recruitment and, to ensure groups started in a timely manner, interventions were started for each group before they were completely filled.
Conclusions
Losing weight and maintaining weight loss is difficult, particularly when quitting smoking since weight gain post-cessation is typical. The current study tested two promising weight management interventions that were previously untested in the context of smoking cessation-related weight gain. Results indicated that weight stability and weight loss interventions are effective for preventing post-smoking cessation weight gain, with the weight loss group having the added benefit of statistically significant weight loss compared to a bibliotherapy group at 12-month follow-up. It will be important in future research to test strategies for integrating this weight loss intervention into publicly available smoking cessation interventions (e.g., state quitlines, Smokefree TXT), particularly for weight concerned smokers.
Data availability
Data will be made available upon reasonable request. Materials are available upon request from R. Krukowski.
References
USDHHS. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Reports of the Surgeon General. Atlanta (GA) 2014.
Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000–2015. Morb Mortality Wkly Rep. 2017;65:1457–64.
Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obes Rev. 2004;5:95–103.
Aubin H-J, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. Br Med J. 2012;345:e4439.
Krukowski RA, Bursac Z, Little MA, Klesges RC. The relationship between body mass index and post-cessation weight gain in the year after quitting smoking: a cross-sectional study. PloS One. 2016;11:e0151290.
Chen S, Kawasaki Y, Hu H, Kuwahara K, Yamamoto M, Uehara A, et al. Smoking cessation, weight gain, and the trajectory of estimated risk of coronary heart disease: 8-year follow-up from a prospective cohort study. Nicotine Tob Res. 2021;23:85–91.
Wang X, Qin LQ, Arafa A, Eshak ES, Hu Y, Dong JY. Smoking cessation, weight gain, cardiovascular risk, and all-cause mortality: a meta-analysis. Nicotine Tob Res. 2021;23:1987–94.
Klesges RC, Brown K, Pascale RW, Murphy M, Williams E, Cigrang JA. Factors associated with participation, attrition, and outcome in a smoking cessation program at the workplace. Health Psychol. 1988;7:575.
Klesges RC, Meyers AW, Klesges LM, LaVasque ME. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychol Bull. 1989;106:204.
Klesges RC, Shumaker SA. Understanding the relations between smoking and body weight and their importance to smoking cessation and relapse. Health Psychol. 1992;11:1.
Jeffery RW, Hennrikus DJ, Lando HA, Murray DM, Liu JW. Reconciling conflicting findings regarding postcessation weight concerns and success in smoking cessation. Health Psychol. 2000;19:242.
Spring B, Howe D, Berendsen M, McFadden HG, Hitchcock K, Rademaker AW, et al. Behavioral intervention to promote smoking cessation and prevent weight gain: a systematic review and meta‐analysis. Addiction. 2009;104:1472–86.
Hartmann-Boyce J, Theodoulou A, Farley A, Hajek P, Lycett D, Jones LL, et al. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev. 2021;10:CD006219.
Wing RR, Tate DF, Espeland MA, Lewis CE, LaRose JG, Gorin AA, et al. Innovative self-regulation strategies to reduce weight gain in young adults: the study of novel approaches to weight gain prevention (SNAP) randomized clinical trial. JAMA Internal Med. 2016;176:755–62.
Kiernan M, Brown SD, Schoffman DE, Lee K, King AC, Taylor CB, et al. Promoting healthy weight with “stability skills first”: A randomized trial. J Consulting Clin Psychol. 2013;81:336.
Unick JL, Beavers D, Bond DS, Clark JM, Jakicic JM, Kitabchi AE, et al.The long-term effectiveness of a lifestyle intervention in severely obese individuals.Am J Med.2013;126:236–42.e2.
Salgado García FI, Derefinko KJ, Bursac Z, Klesges RC, Ebbert JO, Womack CR, et al. Fit & quit: An efficacy trial of two behavioral post-cessation weight gain interventions. Contemporary Clin Trials. 2019;76:31–40.
Harvey-Berino J The Eating Well Diet. Woodstock, VT: Eating Well, Inc.; 2009.
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–27.
Pebley K, Klesges RC, Talcott GW, Kocak M, Krukowski RA. Measurement equivalence of e‐scale and in‐person clinic weights. Obesity. 2019;27:1107–14.
Killi AE, Baspinar MM, Basat O. Association between post-cessation weight gain and eating behavior changes. North Clin İstanb. 2020;7:153.
Tonstad S, Arons C, Rollema H, Berlin I, Hajek P, Fagerström K, et al. Varenicline: mode of action, efficacy, safety and accumulated experience salient for clinical populations. Curr Med Res Opin. 2020;36:713–30.
Klesges RC, Krukowski RA, Klosky JL, Liu W, Srivastava DK, Boyett JM, et al. Efficacy of a tobacco quitline among adult cancer survivors. Prev Med. 2015;73:22–7.
Klesges RC, Krukowski RA, Klosky JL, Liu W, Srivastava DK, Boyett JM, et al. Efficacy of a tobacco quitline among adult survivors of childhood cancer. Nicotine Tob Res. 2014;17:710–8.
Funding
NIDDK R01 DK107747.
Author information
Authors and Affiliations
Contributions
KP was responsible for writing the original draft of the manuscript and editing. ZB was responsible for conducting statistical analyses and editing the manuscript. RCK, JE, CW, JG, ML, KD, and RAK were responsible for providing edits for the manuscript. RAK also secured funding. KD, RAK, RCK, JE, and CW established study procedures and, along with KP and JG, collected study data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pebley, K., Bursac, Z., Klesges, R.C. et al. A randomized controlled trial to reduce post-cessation weight gain. Int J Obes 47, 471–478 (2023). https://doi.org/10.1038/s41366-023-01286-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01286-5