Abstract
Background
Numerous studies have linked visceral adipose tissue (VAT) to gastrointestinal diseases. However, it remains unclear whether these associations reflect causal relationships.
Methods
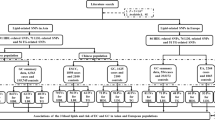
We used a two-sample Mendelian randomization (MR) approach to elucidate the causal effect of VAT on nine non-tumour gastrointestinal diseases. The inverse-variance weighted method was used to perform the MR analyses. Complementary and multivariable MR analyses were performed to confirm the results.
Results
Genetically predicted higher VAT was associated with an increased risk of gastro-oesophageal reflux disease (GORD) (odds ratio [OR], 1.21; 95% confidence interval [CI], 1.09–1.34; P = 3.06 × 10−4), duodenal ulcer (DU) (OR, 1.40; 95% CI, 1.10–1.77; P = 0.005), cholelithiasis (OR, 1.75; 95% CI, 1.53–2.00; P = 1.14 × 10−16), and non-alcoholic fatty liver disease (NAFLD) (OR, 2.68; 95% CI, 1.87–3.82; P = 6.26 × 10−8). There were suggestive associations between VAT and gastric ulcer (GU) (OR, 1.22; 95% CI, 1.01–1.48; P = 0.035) and acute pancreatitis (AP) (OR, 1.26; 95% CI, 1.05–1.52; P = 0.013). However, there was little evidence to support the associations between VAT and inflammatory bowel disease, irritable bowel syndrome, or chronic pancreatitis. The associations with GORD, GU, and NAFLD remained in the multivariable MR analyses with adjustment for body mass index (BMI).
Conclusions
This study provided evidence in support of causal associations between VAT and GORD, GU, DU, cholelithiasis, AP, and NAFLD. Moreover, the associations between GORD, GU, and NAFLD were independent of the effect of BMI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data used in this study were publicly available and can be accessed via the links described in the supplementary material. The R-code used for this study is available from the corresponding author on reasonable request.
References
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–42.
Camilleri M, Malhi H, Acosta A. Gastrointestinal complications of obesity. Gastroenterology. 2017;152:1656–70.
Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts. 2017;10:207–15.
Matsuzawa Y. Adiponectin: a key player in obesity related disorders. Curr Pharm Des. 2010;16:1896–901.
Doyle SL, Donohoe CL, Lysaght J, Reynolds JV. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc Nutr Soc. 2012;71:181–9.
Chung SJ, Kim D, Park MJ, Kim YS, Kim JS, Jung HC, et al. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: a cross-sectional case-control study of 7078 Koreans undergoing health check-ups. Gut. 2008;57:1360–5.
Kim D, Chung GE, Kwak MS, Seo HB, Kang JH, Kim W, et al. Body fat distribution and risk of incident and regressed nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14:132–8.e4.
Uko V, Vortia E, Achkar JP, Karakas P, Fiocchi C, Worley S, et al. Impact of abdominal visceral adipose tissue on disease outcome in pediatric Crohn’s disease. Inflamm Bowel Dis. 2014;20:2286–91.
Natu A, Stevens T, Kang L, Yasinow S, Mansoor E, Lopez R, et al. Visceral adiposity predicts severity of acute pancreatitis. Pancreas. 2017;46:776–81.
Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30:543–52.
Karlsson T, Rask-Andersen M, Pan G, Höglund J, Wadelius C, Ek WE, et al. Contribution of genetics to visceral adiposity and its relation to cardiovascular and metabolic disease. Nat Med. 2019;25:1390–5.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408.
Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755–64.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36:1783–802.
Bowden J, Davey, Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8.
Hoffmann TJ, Choquet H, Yin J, Banda Y, Kvale MN, Glymour M, et al. A large multiethnic genome-wide association study of adult body mass index identifies novel loci. Genetics. 2018;210:499–515.
Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2019;48:713–27.
Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46:1734–9.
Sanderson E, Spiller W, Bowden J. Testing and correcting for weak and pleiotropic instruments in two-sample multivariable Mendelian randomization. Stat Med. 2021;40:5434–52.
El-Serag H. Role of obesity in GORD-related disorders. Gut. 2008;57:281–4.
Nam SY, Choi IJ, Ryu KH, Park BJ, Kim HB, Nam BH. Abdominal visceral adipose tissue volume is associated with increased risk of erosive esophagitis in men and women. Gastroenterology. 2010;139:1902–11.e2.
El-Serag HB, Hashmi A, Garcia J, Richardson P, Alsarraj A, Fitzgerald S, et al. Visceral abdominal obesity measured by CT scan is associated with an increased risk of Barrett’s oesophagus: a case-control study. Gut. 2014;63:220–9.
Pandolfino JE. The relationship between obesity and GERD: “big or overblown”. Am J Gastroenterol. 2008;103:1355–7.
Pandolfino JE, El-Serag HB, Zhang Q, Shah N, Ghosh SK, Kahrilas PJ. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology. 2006;130:639–49.
Boylan MR, Khalili H, Huang ES, Chan AT. Measures of adiposity are associated with increased risk of peptic ulcer. Clin Gastroenterol Hepatol. 2014;12:1688–94.
Kim HJ, Yoo TW, Park DI, Park JH, Cho YK, Sohn CI, et al. Influence of overweight and obesity on upper endoscopic findings. J Gastroenterol Hepatol. 2007;22:477–81.
Tagawa N, Fujinami A, Natsume S, Mizuno S, Kato I. Relationship between adiponectin multimer levels and subtypes of cerebral infarction. PLoS One. 2022;17:e0262542.
Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–7.
Erhayiem B, Dhingsa R, Hawkey CJ, Subramanian V. Ratio of visceral to subcutaneous fat area is a biomarker of complicated Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9:684–7.e1.
Van Der Sloot KW, Joshi AD, Bellavance DR, Gilpin KK, Stewart KO, Lochhead P, et al. Visceral adiposity, genetic susceptibility, and risk of complications among individuals with Crohn’s disease. Inflamm Bowel Dis. 2017;23:82–8.
Grillot J, D’Engremont C, Parmentier AL, Lakkis Z, Piton G, Cazaux D, et al. Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn’s disease. Clin Nutr. 2020;39:3024–30.
Büning C, von Kraft C, Hermsdorf M, Gentz E, Wirth EK, Valentini L, et al. Visceral adipose tissue in patients with Crohn’s disease correlates with disease activity, inflammatory markers, and outcome. Inflamm Bowel Dis. 2015;21:2590–7.
Lee CG, Lee JK, Kang YS, Shin S, Kim JH, Lim YJ, et al. Visceral abdominal obesity is associated with an increased risk of irritable bowel syndrome. Am J Gastroenterol. 2015;110:310–9.
Sekine K, Nagata N, Sakamoto K, Arai T, Shimbo T, Shinozaki M, et al. Abdominal visceral fat accumulation measured by computed tomography associated with an increased risk of gallstone disease. J Gastroenterol Hepatol. 2015;30:1325–31.
Zhang Y, Chen TW, Zhang XM, Wang YX, Chi XX, Li XH, et al. Abdominal regional fat distribution on MRI correlates with cholecystolithiasis. PLoS One. 2014;9:e109776.
Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–14.
Berr F, Mayer M, Sackmann MF, Sauerbruch T, Holl J, Paumgartner G. Pathogenic factors in early recurrence of cholesterol gallstones. Gastroenterology. 1994;106:215–24.
Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Macronutrients and insulin resistance in cholesterol gallstone disease. Am J Gastroenterol. 2008;103:2932–9.
Bükülmez A, Özer Gökaslan Ç, Oflu AT. Increase in visceral adipose tissue and subcutaneous adipose tissue thickness in children with acute pancreatitis. A case-control study. Arch Pediatr. 2021;28:29–32.
Yashima Y, Isayama H, Tsujino T, Nagano R, Yamamoto K, Mizuno S, et al. A large volume of visceral adipose tissue leads to severe acute pancreatitis. J Gastroenterol. 2011;46:1213–8.
Kuan LL, Dennison AR, Garcea G. Association of visceral adipose tissue on the incidence and severity of acute pancreatitis: A systematic review. Pancreatology. 2020;20:1056–61.
Lee S, Kim KW, Lee J, Park T, Khang S, Jeong H, et al. Visceral adiposity as a risk factor for lean non-alcoholic fatty liver disease in potential living liver donors. J Gastroenterol Hepatol. 2021;36:3212–8.
Czaja MJ. Liver injury in the setting of steatosis: crosstalk between adipokine and cytokine. Hepatology. 2004;40:19–22.
Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54.
Schooling CM, Lopez PM, Yang Z, Zhao JV, Au Yeung SL, Huang JV. Use of multivariable mendelian randomization to address biases due to competing risk before recruitment. Front Genet. 2020;11:610852.
Yu B, Sun Y, Du X, Zhang H, Chen C, Tan X. et al. Age-specific and sex-specific associations of visceral adipose tissue mass and fat-to-muscle mass ratio with risk of mortality. J Cachexia Sarcopenia Muscle. 2023;14:406–417.
Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne CD, Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism. 2020;111s:154170.
Persson PG, Bernell O, Leijonmarck CE, Farahmand BY, Hellers G, Ahlbom A. Survival and cause-specific mortality in inflammatory bowel disease: a population-based cohort study. Gastroenterology. 1996;110:1339–45.
Acknowledgements
We want to acknowledge all the participants and investigators of the GWAS involved in the present study for generously sharing the summary-level data.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81873484).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analyses were performed by Xingang Sun and Yifan Yuan. The first draft of the manuscript was written by Xingang Sun, Yifan Yuan, and Lu Chen. Liangrong Zheng and Mei Ye reviewed the final manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
We used summary-level data from published studies and publicly available GWAS with ethical approval obtained from their respective institutional review boards and informed consent provided by their participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, X., Yuan, Y., Chen, L. et al. Genetically predicted visceral adipose tissue and risk of nine non-tumour gastrointestinal diseases: evidence from a Mendelian randomization study. Int J Obes 47, 406–412 (2023). https://doi.org/10.1038/s41366-023-01279-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01279-4