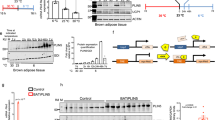
Abstract
Exposure to low ambient temperatures has previously been demonstrated to markedly improve glucose homeostasis in both rodents and humans. Although the brown adipose tissue is key in mediating these beneficial effects in rodents, its contribution appears more limited in humans. Hence, the exact tissues and underlying mechanisms that mediate cold-induced improvements in glucose homeostasis in humans remain to be fully established. In this review, we evaluated the response of the main organs involved in glucose metabolism (i.e. pancreas, liver, (white) adipose tissue, and skeletal muscle) to cold exposure and discuss their potential contribution to cold-induced improvements in glucose homeostasis in humans. We here show that cold exposure has widespread effects on metabolic organs involved in glucose regulation. Nevertheless, cold-induced improvements in glucose homeostasis appear primarily mediated via adaptations within the skeletal muscle and (presumably) white adipose tissue. Since the underlying mechanisms remain elusive, future studies should be aimed at pinpointing the exact physiological and molecular mechanisms involved in humans. Nonetheless, cold exposure holds great promise as a novel, additive lifestyle approach to improve glucose homeostasis in insulin resistant individuals.

Parts of this graphical abstract were created using (modified) images from Servier Medical Art, licensed under the Creative Commons Attribution 3.0 Unported License. TG = thermogenesis, TAG = triacylglycerol, FFA = free fatty acid, SLN = sarcolipin, UCP3 = uncoupling protein 3, β2-AR = beta-2 adrenergic receptor, SNS = sympathetic nervous system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed for the present review.
References
Dulloo AG, Young JB, Landsberg L. Sympathetic nervous system responses to cold exposure and diet in rat skeletal muscle. Am J Physiol. 1988;255:E180–8. 2 Pt 1
Depocas F, Behrens WA. Levels of noradrenaline in plasma during thermogenesis induced by cold-exposure or by noradrenaline infusion in warm- and in cold-acclimated rats. Experientia Suppl. 1978;32:135–46.
Picotti GB, Carruba MO, Ravazzani C, Cesura AM, Galva MD, Da Prada M. Plasma catecholamines in rats exposed to cold: effects of ganglionic and adrenoreceptor blockade. Eur J Pharmacol. 1981;69:321–9.
Nakamura K, Morrison SF. Central efferent pathways for cold-defensive and febrile shivering. J Physiol. 2011;589:3641–58. Pt 14
Haman F, Blondin DP. Shivering thermogenesis in humans: origin, contribution and metabolic requirement. Temperature. 2017;4:217–26.
van Marken Lichtenbelt WD, Schrauwen P. Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R285–96.
Vallerand AL, Lupien J, Bukowiecki LJ. Interactions of cold exposure and starvation on glucose tolerance and insulin response. Am J Physiol. 1983;245:E575–81.
Smith OL, Davidson SB. Shivering thermogenesis and glucose uptake by muscles of normal or diabetic rats. Am J Physiol. 1982;242:R109–15.
Smith OL. Insulin response in rats acutely exposed to cold. Can J Physiol Pharmacol. 1984;62:924–7.
Vallerand AL, Perusse F, Bukowiecki LJ. Cold exposure potentiates the effect of insulin on in vivo glucose uptake. Am J Physiol. 1987;253:E179–86. 2 Pt 1
Vallerand AL, Perusse F, Bukowiecki LJ. Stimulatory effects of cold exposure and cold acclimation on glucose uptake in rat peripheral tissues. Am J Physiol. 1990;259:R1043–9. 5 Pt 2
Vallerand AL, Lupien J, Bukowiecki LJ. Cold exposure reverses the diabetogenic effects of high-fat feeding. Diabetes. 1986;35:329–34.
Cunningham JJ, Gulino MA, Meara PA, Bode HH. Enhanced hepatic insulin sensitivity and peripheral glucose uptake in cold acclimating rats. Endocrinology. 1985;117:1585–9.
Vallerand AL, Zamecnik J, Jacobs I. Plasma glucose turnover during cold stress in humans. J Appl Physiol. 1995;78:1296–302.
Vallerand AL, Frim J, Kavanagh MF. Plasma glucose and insulin responses to oral and intravenous glucose in cold-exposed humans. J Appl Physiol. 1988;65:2395–9.
Iwen KA, Backhaus J, Cassens M, Waltl M, Hedesan OC, Merkel M, et al. Cold-induced brown adipose tissue activity alters plasma fatty acids and improves glucose metabolism in men. J Clin Endocrinol Metab. 2017;102:4226–34.
Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med. 2015;21:863–5.
Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359.
Shibata H, Perusse F, Vallerand A, Bukowiecki LJ. Cold exposure reverses inhibitory effects of fasting on peripheral glucose uptake in rats. Am J Physiol. 1989;257:R96–101. 1 Pt 2
Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–9.
Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–5.
Xiao C, Goldgof M, Gavrilova O, Reitman ML. Anti-obesity and metabolic efficacy of the beta3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22 degrees C. Obesity. 2015;23:1450–9.
Olsen JM, Csikasz RI, Dehvari N, Lu L, Sandstrom A, Oberg AI, et al. beta3-Adrenergically induced glucose uptake in brown adipose tissue is independent of UCP1 presence or activity: mediation through the mTOR pathway. Mol Metab. 2017;6:611–9.
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8.
Brendle C, Werner MK, Schmadl M, la Fougere C, Nikolaou K, Stefan N, et al. Correlation of brown adipose tissue with other body fat compartments and patient characteristics: a retrospective analysis in a large patient cohort using PET/CT. Acad Radiol. 2018;25:102–10.
Blondin DP, Labbe SM, Noll C, Kunach M, Phoenix S, Guerin B, et al. Selective impairment of glucose but not fatty acid or oxidative metabolism in brown adipose tissue of subjects with type 2 diabetes. Diabetes. 2015;64:2388–97.
Roder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48:e219.
Seitz HJ, Krone W, Wilke H, Tarnowski W. Rapid rise in plasma glucagon induced by acute cold exposure in man and rat. Pflugers Arch. 1981;389:115–20.
Kuroshima A, Yahata T, Ohno T. Changes in plasma glucagon levels to stressful environmental temperatures. Jpn J Physiol. 1981;31:43–52.
Kuroshima A, Yahata T, Doi K, Ohno T. Thermal and metabolic responses of temperature-acclimated rats during cold and heat exposures. Jpn J Physiol. 1982;32:561–71.
Sepa-Kishi DM, Katsnelson G, Bikopoulos G, Iqbal A, Ceddia RB. Cold acclimation reduces hepatic protein Kinase B and AMP-activated protein kinase phosphorylation and increases gluconeogenesis in Rats. Physiol Rep. 2018;6:e13592.
Doi K, Ohno T, Kuroshima A. Role of endocrine pancreas in temperature acclimation. Life Sci. 1982;30:2253–9.
Morton GJ, Muta K, Kaiyala KJ, Rojas JM, Scarlett JM, Matsen ME, et al. Evidence that the sympathetic nervous system elicits rapid, coordinated, and reciprocal adjustments of insulin secretion and insulin sensitivity during cold exposure. Diabetes. 2017;66:823–34.
Gasparetti AL, de Souza CT, Pereira-da-Silva M, Oliveira RL, Saad MJ, Carneiro EM, et al. Cold exposure induces tissue-specific modulation of the insulin-signalling pathway in Rattus norvegicus. J Physiol. 2003;552:149–62. Pt 1
Beck LV, Zaharko DS, Kalser SC. Variation in serum insulin and glucose of rats with chronic cold exposure. Life Sci. 1967;6:1501–6.
Harada E, Habara Y, Kanno T. Cold acclimation in insulin secretion of isolated perfused pancreas of the rat. Am J Physiol. 1982;242:E360–7.
Hanssen MJ, van der Lans AA, Brans B, Hoeks J, Jardon KM, Schaart G, et al. Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes. 2016;65:1179–89.
Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–99.
Sellers AJ, Pallubinsky H, Rense P, Bijnens W, van de Weijer T, Moonen-Kornips E, et al. The effect of cold exposure with shivering on glucose tolerance in healthy men. J Appl Physiol. 2021;130:193–205.
Habara Y, Ohno T, Yahata T, Kuroshima A. Effects of adrenal demedullation combined with chemical sympathectomy on cold-induced responses of endocrine pancreas in rats. Experientia. 1983;39:399–400.
Young JB, Landsberg L. Effect of diet and cold exposure on norepinephrine turnover in pancreas and liver. Am J Physiol. 1979;236:E524–33.
van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123:3395–403.
Remie CME, Moonen MPB, Roumans KHM, Nascimento EBM, Gemmink A, Havekes B, et al. Metabolic responses to mild cold acclimation in type 2 diabetes patients. Nat Commun. 2021;12:1516.
Grefhorst A, van den Beukel JC, Dijk W, Steenbergen J, Voortman GJ, Leeuwenburgh S, et al. Multiple effects of cold exposure on livers of male mice. J Endocrinol. 2018;238:91–106.
Bobbioni-Harsch E, Assimacopoulos-Jeannet F, Jeanrenaud B. Modifications of glucose and lipid metabolism in cold-acclimated lean and genetically obese rats. J Appl Physiol. 1994;76:1106–12.
Guezennec CY, Nonglaton J, Serrurier B, Merino D, Defer G. Hormonal and metabolic response to physical exercise, fasting and cold exposure in the rat. Effects on ketogenesis in isolated hepatocytes. Eur J Appl Physiol Occup Physiol. 1988;57:114–9.
Penner PE, Himms-Hagen J. Gluconeogenesis in rats during cold acclimation. Can J Biochem. 1968;46:1205–13.
Nakagawa H, Nagai K. Cold adaptation. I. Effect of cold-exposure on gluconeogenesis. J Biochem. 1971;69:923–34.
Smith SA, Young P, Cawthorne MA. Quantification in vivo of the effects of insulin on glucose utilization in individual tissues of warm- and cold-acclimated rats. Biochem J. 1986;237:789–95.
Johnstone AM, Murison SD, Duncan JS, Rance KA, Speakman JR. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr. 2005;82:941–8.
Blondin DP, Nielsen S, Kuipers EN, Severinsen MC, Jensen VH, Miard S, et al. Human brown adipocyte thermogenesis is driven by beta2-AR stimulation. Cell Metab. 2020;32:287–300. e7
Granneman JG, Burnazi M, Zhu Z, Schwamb LA. White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab. 2003;285:E1230–6.
Liu X, Perusse F, Bukowiecki LJ. Chronic norepinephrine infusion stimulates glucose uptake in white and brown adipose tissues. Am J Physiol. 1994;266:R914–20. 3 Pt 2
Castro E, Vieira TS, Oliveira TE, Ortiz-Silva M, Andrade ML, Tomazelli CA, et al. Adipocyte-specific mTORC2 deficiency impairs BAT and iWAT thermogenic capacity without affecting glucose uptake and energy expenditure in cold-acclimated mice. Am J Physiol Endocrinol Metab. 2021;321:E592–E605.
Agosto E, Cimmino M, Minaire Y, Geloen A. Short-term cold-exposure does not improve insulin sensitivity in rats. Comp Biochem Physiol A Physiol. 1997;117:231–8.
Walden TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab. 2012;302:E19–31.
Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407.
Sepa-Kishi DM, Jani S, Da Eira D, Ceddia RB. Cold acclimation enhances UCP1 content, lipolysis, and triacylglycerol resynthesis, but not mitochondrial uncoupling and fat oxidation, in rat white adipocytes. Am J Physiol Cell Physiol. 2019;316:C365–C76.
Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103:931–42. Pt 4
Okamatsu-Ogura Y, Fukano K, Tsubota A, Uozumi A, Terao A, Kimura K, et al. Thermogenic ability of uncoupling protein 1 in beige adipocytes in mice. PLoS One. 2013;8:e84229.
Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013;5:1196–203.
Young P, Arch JR, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 1984;167:10–4.
Wang Z, Ning T, Song A, Rutter J, Wang QA, Jiang L. Chronic cold exposure enhances glucose oxidation in brown adipose tissue. EMBO Rep. 2020;21:e50085.
Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–76.
Mossenbock K, Vegiopoulos A, Rose AJ, Sijmonsma TP, Herzig S, Schafmeier T. Browning of white adipose tissue uncouples glucose uptake from insulin signaling. PLoS One. 2014;9:e110428.
Jia XW, Fang DL, Shi XY, Lu T, Yang C, Gao Y. Inducible beige adipocytes improve impaired glucose metabolism in interscapular BAT-removal mice. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866:158871.
Finlin BS, Memetimin H, Confides AL, Kasza I, Zhu B, Vekaria HJ, et al. Human adipose beiging in response to cold and mirabegron. JCI Insight. 2018;3:e121510.
Sidossis LS, Porter C, Saraf MK, Borsheim E, Radhakrishnan RS, Chao T, et al. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. 2015;22:219–27.
Finlin BS, Memetimin H, Zhu B, Confides AL, Vekaria HJ, El Khouli RH, et al. The beta3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J Clin Invest. 2020;130:2319–31.
Meyer CW, Willershauser M, Jastroch M, Rourke BC, Fromme T, Oelkrug R, et al. Adaptive thermogenesis and thermal conductance in wild-type and UCP1-KO mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1396–406.
Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/- mice. J Biol Chem. 2006;281:31894–908.
Roesler A, Kazak L. UCP1-independent thermogenesis. Biochem J. 2020;477:709–25.
Chouchani ET, Kazak L, Spiegelman BM. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 2019;29:27–37.
Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163:643–55.
Kazak L, Chouchani ET, Lu GZ, Jedrychowski MP, Bare CJ, Mina AI, et al. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab. 2017;26:693.
Rahbani JF, Roesler A, Hussain MF, Samborska B, Dykstra CB, Tsai L, et al. Creatine kinase B controls futile creatine cycling in thermogenic fat. Nature. 2021;590:480–5.
Brownstein AJ, Veliova M, Acin-Perez R, Liesa M, Shirihai OS. ATP-consuming futile cycles as energy dissipating mechanisms to counteract obesity. Rev Endocr Metab Disord. 2022;23:121–31.
Flachs P, Rossmeisl M, Kuda O, Kopecky J. Stimulation of mitochondrial oxidative capacity in white fat independent of UCP1: a key to lean phenotype. Biochim Biophys Acta. 2013;1831:986–1003.
Hammond VA, Johnston DG. Substrate cycling between triglyceride and fatty acid in human adipocytes. Metabolism. 1987;36:308–13.
Bottcher H, Furst P. Decreased white fat cell thermogenesis in obese individuals. Int J Obes Relat Metab Disord. 1997;21:439–44.
Wolfe RR, Klein S, Carraro F, Weber JM. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am J Physiol. 1990;258:E382–9. 2 Pt 1
Flachs P, Adamcova K, Zouhar P, Marques C, Janovska P, Viegas I, et al. Induction of lipogenesis in white fat during cold exposure in mice: link to lean phenotype. Int J Obes. 2017;41:997.
Mottillo EP, Balasubramanian P, Lee YH, Weng C, Kershaw EE, Granneman JG. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic beta3-adrenergic receptor activation. J Lipid Res. 2014;55:2276–86.
Vallerand AL, Zamecnik J, Jones PJ, Jacobs I. Cold stress increases lipolysis, FFA Ra and TG/FFA cycling in humans. Aviat Space Environ Med. 1999;70:42–50.
DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985;76:149–55.
DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–7.
Blondin DP, Labbe SM, Phoenix S, Guerin B, Turcotte EE, Richard D, et al. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol. 2015;593:701–14.
Oliveira RL, Ueno M, de Souza CT, Pereira-da-Silva M, Gasparetti AL, Bezzera RM, et al. Cold-induced PGC-1alpha expression modulates muscle glucose uptake through an insulin receptor/Akt-independent, AMPK-dependent pathway. Am J Physiol Endocrinol Metab. 2004;287:E686–95.
Blondin DP, Daoud A, Taylor T, Tingelstad HC, Bezaire V, Richard D, et al. Four-week cold acclimation in adult humans shifts uncoupling thermogenesis from skeletal muscles to brown adipose tissue. J Physiol. 2017;595:2099–113.
Wijers SL, Schrauwen P, Saris WH, van Marken, Lichtenbelt WD. Human skeletal muscle mitochondrial uncoupling is associated with cold induced adaptive thermogenesis. PLoS One. 2008;3:e1777.
Wijers SL, Schrauwen P, van Baak MA, Saris WH, van Marken Lichtenbelt WD. Beta-adrenergic receptor blockade does not inhibit cold-induced thermogenesis in humans: possible involvement of brown adipose tissue. J Clin Endocrinol Metab. 2011;96:E598–605.
Schrauwen P, Westerterp-Plantenga MS, Kornips E, Schaart G, van Marken Lichtenbelt WD. The effect of mild cold exposure on UCP3 mRNA expression and UCP3 protein content in humans. Int J Obes Relat Metab Disord. 2002;26:450–7.
Blondin DP, Haman F. Shivering and nonshivering thermogenesis in skeletal muscles. Handb Clin Neurol. 2018;156:153–73.
Samec S, Seydoux J, Dulloo AG. Role of UCP homologues in skeletal muscles and brown adipose tissue: mediators of thermogenesis or regulators of lipids as fuel substrate? FASEB J. 1998;12:715–24.
Schrauwen P, Hesselink MK. The role of uncoupling protein 3 in fatty acid metabolism: protection against lipotoxicity? Proc Nutr Soc. 2004;63:287–92.
Pant M, Bal NC, Periasamy M. Sarcolipin: a key thermogenic and metabolic regulator in skeletal muscle. Trends Endocrinol Metab. 2016;27:881–92.
Maurya SK, Herrera JL, Sahoo SK, Reis FCG, Vega RB, Kelly DP, et al. Sarcolipin signaling promotes mitochondrial biogenesis and oxidative metabolism in skeletal muscle. Cell Rep. 2018;24:2919–31.
Mall S, Broadbridge R, Harrison SL, Gore MG, Lee AG, East JM. The presence of sarcolipin results in increased heat production by Ca(2+)-ATPase. J Biol Chem. 2006;281:36597–602.
Bal NC, Maurya SK, Singh S, Wehrens XH, Periasamy M. Increased reliance on muscle-based thermogenesis upon acute minimization of brown adipose tissue function. J Biol Chem. 2016;291:17247–57.
Rowland LA, Bal NC, Kozak LP, Periasamy M. Uncoupling protein 1 and sarcolipin are required to maintain optimal thermogenesis, and loss of both systems compromises survival of mice under cold stress. J Biol Chem. 2015;290:12282–9.
Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18:1575–9.
Paran CW, Verkerke AR, Heden TD, Park S, Zou K, Lawson HA, et al. Reduced efficiency of sarcolipin-dependent respiration in myocytes from humans with severe obesity. Obesity. 2015;23:1440–9.
Maurya SK, Bal NC, Sopariwala DH, Pant M, Rowland LA, Shaikh SA, et al. Sarcolipin is a key determinant of the basal metabolic rate, and its overexpression enhances energy expenditure and resistance against diet-induced obesity. J Biol Chem. 2015;290:10840–9.
Bombardier E, Smith IC, Vigna C, Fajardo VA, Tupling AR. Ablation of sarcolipin decreases the energy requirements for Ca2+ transport by sarco(endo)plasmic reticulum Ca2+-ATPases in resting skeletal muscle. FEBS Lett. 2013;587:1687–92.
Periasamy M, Herrera JL, Reis FCG. Skeletal muscle thermogenesis and its role in whole body energy metabolism. Diabetes Metab J. 2017;41:327–36.
Morales-Alamo D, Martinez-Canton M, Gelabert-Rebato M, Martin-Rincon M, de Pablos-Velasco P, Holmberg HC, et al. Sarcolipin expression in human skeletal muscle: influence of energy balance and exercise. Scand J Med Sci Sports. 2020;30:408–20.
Eyolfson DA, Tikuisis P, Xu X, Weseen G, Giesbrecht GG. Measurement and prediction of peak shivering intensity in humans. Eur J Appl Physiol. 2001;84:100–6.
Haman F, Peronnet F, Kenny GP, Massicotte D, Lavoie C, Scott C, et al. Effect of cold exposure on fuel utilization in humans: plasma glucose, muscle glycogen, and lipids. J Appl Physiol. 2002;93:77–84.
Horvath SM. Exercise in a cold environment. Exerc Sport Sci Rev. 1981;9:221–63.
Jamerson KA, Smith SD, Amerena JV, Grant E, Julius S. Vasoconstriction with norepinephrine causes less forearm insulin resistance than a reflex sympathetic vasoconstriction. Hypertension. 1994;23:1006–11. 6 Pt 2
Wasserman DH, Ayala JE. Interaction of physiological mechanisms in control of muscle glucose uptake. Clin Exp Pharmacol Physiol. 2005;32:319–23.
Shimazu T, Sudo M, Minokoshi Y, Takahashi A. Role of the hypothalamus in insulin-independent glucose uptake in peripheral tissues. Brain Res Bull. 1991;27:501–4.
Sudo M, Minokoshi Y, Shimazu T. Ventromedial hypothalamic stimulation enhances peripheral glucose uptake in anesthetized rats. Am J Physiol. 1991;261:E298–303. 3 Pt 1
Minokoshi Y, Okano Y, Shimazu T. Regulatory mechanism of the ventromedial hypothalamus in enhancing glucose uptake in skeletal muscles. Brain Res. 1994;649:343–7.
Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci USA. 2012;109:10001–5.
Scriven AJ, Brown MJ, Murphy MB, Dollery CT. Changes in blood pressure and plasma catecholamines caused by tyramine and cold exposure. J Cardiovasc Pharmacol. 1984;6:954–60.
O’Malley BP, Cook N, Richardson A, Barnett DB, Rosenthal FD. Circulating catecholamine, thyrotrophin, thyroid hormone and prolactin responses of normal subjects to acute cold exposure. Clin Endocrinol. 1984;21:285–91.
Molinoff PB. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs. 1984;28:1–15.
Baker JG. The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol. 2010;160:1048–61.
Nevzorova J, Bengtsson T, Evans BA, Summers RJ. Characterization of the beta-adrenoceptor subtype involved in mediation of glucose transport in L6 cells. Br J Pharmacol. 2002;137:9–18.
Liggett SB, Shah SD, Cryer PE. Characterization of beta-adrenergic receptors of human skeletal muscle obtained by needle biopsy. Am J Physiol. 1988;254:E795–8. 6 Pt 1
Sato M, Dehvari N, Oberg AI, Dallner OS, Sandstrom AL, Olsen JM, et al. Improving type 2 diabetes through a distinct adrenergic signaling pathway involving mTORC2 that mediates glucose uptake in skeletal muscle. Diabetes. 2014;63:4115–29.
Kalinovich A, Dehvari N, Åslund A, van Beek S, Halleskog C, Olsen J, et al. Treatment with a β-2-adrenoceptor agonist stimulates glucose uptake in skeletal muscle and improves glucose homeostasis, insulin resistance and hepatic steatosis in mice with diet-induced obesity. Diabetologia. 2020;63:1603–15.
Nevzorova J, Evans BA, Bengtsson T, Summers RJ. Multiple signalling pathways involved in beta2-adrenoceptor-mediated glucose uptake in rat skeletal muscle cells. Br J Pharmacol. 2006;147:446–54.
Tanishita T, Shimizu Y, Minokoshi Y, Shimazu T. The beta3-adrenergic agonist BRL37344 increases glucose transport into L6 myocytes through a mechanism different from that of insulin. J Biochem. 1997;122:90–5.
Mukaida S, Sato M, Oberg AI, Dehvari N, Olsen JM, Kocan M, et al. BRL37344 stimulates GLUT4 translocation and glucose uptake in skeletal muscle via beta2-adrenoceptors without causing classical receptor desensitization. Am J Physiol Regul Integr Comp Physiol. 2019;316:R666–R77.
Abe H, Minokoshi Y, Shimazu T. Effect of a beta 3-adrenergic agonist, BRL35135A, on glucose uptake in rat skeletal muscle in vivo and in vitro. J Endocrinol. 1993;139:479–86.
Liu YL, Cawthorne MA, Stock MJ. Biphasic effects of the beta-adrenoceptor agonist, BRL 37344, on glucose utilization in rat isolated skeletal muscle. Br J Pharmacol. 1996;117:1355–61.
Philipson LH. beta-Agonists and metabolism. J Allergy Clin Immunol. 2002;110:S313–7.
Guhan AR, Cooper S, Oborne J, Lewis S, Bennett J, Tattersfield AE. Systemic effects of formoterol and salmeterol: a dose-response comparison in healthy subjects. Thorax. 2000;55:650–6.
Burgess C, Ayson M, Rajasingham S, Crane J, Della Cioppa G, Till MD. The extrapulmonary effects of increasing doses of formoterol in patients with asthma. Eur J Clin Pharmacol. 1998;54:141–7.
Liu YL, Stock MJ. Acute effects of the beta 3-adrenoceptor agonist, BRL 35135, on tissue glucose utilisation. Br J Pharmacol. 1995;114:888–94.
Smith SA, Levy AL, Sennitt MV, Simson DL, Cawthorne MA. Effects of BRL 26830, a novel beta-adrenoceptor agonist, on glucose tolerance, insulin sensitivity and glucose turnover in Zucker (fa/fa) rats. Biochem Pharmacol. 1985;34:2425–9.
Castle A, Yaspelkis BB 3rd, Kuo CH, Ivy JL. Attenuation of insulin resistance by chronic beta2-adrenergic agonist treatment possible muscle specific contributions. Life Sci. 2001;69:599–611.
Torgan CE, Brozinick JT Jr, Banks EA, Cortez MY, Wilcox RE, Ivy JL. Exercise training and clenbuterol reduce insulin resistance of obese Zucker rats. Am J Physiol. 1993;264:E373–9. 3 Pt 1
van Beek S, Kalinovich A, Schaart G, Bengtsson T, Hoeks J. Prolonged beta2-adrenergic agonist treatment improves glucose homeostasis in diet-induced obese UCP1(-/-) mice. Am J Physiol Endocrinol Metab. 2021;320:E619–628.
Pan SJ, Hancock J, Ding Z, Fogt D, Lee M, Ivy JL. Effects of clenbuterol on insulin resistance in conscious obese Zucker rats. Am J Physiol Endocrinol Metab. 2001;280:E554–61.
Meister J, Bone DBJ, Knudsen JR, Barella LF, Velenosi TJ, Akhmedov D, et al. Clenbuterol exerts antidiabetic activity through metabolic reprogramming of skeletal muscle cells. Nat Commun. 2022;13:22.
Elayan H, Milic M, Sun P, Gharaibeh M, Ziegler MG. Chronic beta2 adrenergic agonist, but not exercise, improves glucose handling in older type 2 diabetic mice. Cell Mol Neurobiol. 2012;32:871–7.
Kalinovich A, Dehvari N, Aslund A, van Beek S, Halleskog C, Olsen J, et al. Treatment with a beta-2-adrenoceptor agonist stimulates glucose uptake in skeletal muscle and improves glucose homeostasis, insulin resistance and hepatic steatosis in mice with diet-induced obesity. Diabetologia. 2020;63:1603–15.
Onslev J, Jensen J, Bangsbo J, Wojtaszewski J, Hostrup M. beta2-agonist induces net leg glucose uptake and free fatty acid release at rest but not during exercise in young men. J Clin Endocrinol Metab. 2019;104:647–57.
Massara F, Fassio V, Camanni F, Martina V, Molinatti G. Some metabolic and hormonal effects of salbutamol in man. Acta Diabetol Lat. 1976;13:146–53.
Kendall MJ, Dean S, Bradley D, Gibson R, Worthington DJ. Cardiovascular and metabolic effects of terbutaline. J Clin Hosp Pharm. 1982;7:31–6.
Mitchell TH, Ellis RD, Smith SA, Robb G, Cawthorne MA. Effects of BRL 35135, a beta-adrenoceptor agonist with novel selectivity, on glucose tolerance and insulin sensitivity in obese subjects. Int J Obes. 1989;13:757–66.
Scheidegger K, Robbins DC, Danforth E Jr. Effects of chronic beta receptor stimulation on glucose metabolism. Diabetes. 1984;33:1144–9.
Jessen S, Baasch-Skytte T, Onslev J, Eibye K, Backer V, Bangsbo J, et al. Muscle hypertrophic effect of inhaled beta2 -agonist is associated with augmented insulin-stimulated whole-body glucose disposal in young men. J Physiol. 2022;600:2345–57.
Rodriguez A, Becerril S, Ezquerro S, Mendez-Gimenez L, Fruhbeck G. Crosstalk between adipokines and myokines in fat browning. Acta Physiol. 2017;219:362–81.
Scheja L, Heeren J. Metabolic interplay between white, beige, brown adipocytes and the liver. J Hepatol. 2016;64:1176–86.
Krapf S, Schjolberg T, Asoawe L, Honkanen SK, Kase ET, Thoresen GH, et al. Novel methods for cold exposure of skeletal muscle in vivo and in vitro show temperature-dependent myokine production. J Therm Biol. 2021;98:102930.
Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–23.
Lee P, Brychta RJ, Linderman J, Smith S, Chen KY, Celi FS. Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J Clin Endocrinol Metab. 2013;98:E98–102.
Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–9.
Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286:12983–90.
Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–81.
Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–35.
Wente W, Efanov AM, Brenner M, Kharitonenkov A, Koster A, Sandusky GE, et al. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55:2470–8.
Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63:514–25.
Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes. 2014;38:1538–44.
Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EB, van der Lans AA, et al. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obes. 2015;39:1696–702.
Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, et al. The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–49.
Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63:3686–98.
Speakman JR, Heidari-Bakavoli S. Type 2 diabetes, but not obesity, prevalence is positively associated with ambient temperature. Sci Rep. 2016;6:30409.
Soberg S, Lofgren J, Philipsen FE, Jensen M, Hansen AE, Ahrens E, et al. Altered brown fat thermoregulation and enhanced cold-induced thermogenesis in young, healthy, winter-swimming men. Cell Rep Med. 2021;2:100408.
Ivanova YM, Blondin DP. Examining the benefits of cold exposure as a therapeutic strategy for obesity and type 2 diabetes. J Appl Physiol. 2021;130:1448–59.
Miyai N, Arita M, Miyashita K, Morioka I, Shiraishi T, Nishio I. Blood pressure response to heart rate during exercise test and risk of future hypertension. Hypertension. 2002;39:761–6.
Funding
SvB is supported by a grant from the Nutrim NWO graduate program.
Author information
Authors and Affiliations
Contributions
SvB and JH conceived the idea and designed the review. SvB performed the literature search and drafted the manuscript. TB and DH contributed to the further development of the review. All authors revised the manuscript and gave approval for the final version to be published.
Corresponding author
Ethics declarations
Competing interests
TB owns stock in Atrogi A.B. Other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Beek, S., Hashim, D., Bengtsson, T. et al. Physiological and molecular mechanisms of cold-induced improvements in glucose homeostasis in humans beyond brown adipose tissue. Int J Obes 47, 338–347 (2023). https://doi.org/10.1038/s41366-023-01270-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01270-z