Abstract
Background and objectives
A2A adenosine receptor (A2AAR)-mediated signaling in adipose tissues has been investigated as a potential target for obesity-related metabolic diseases. LJ-4378 has been developed as a dual-acting ligand with A2AAR agonist and A3 adenosine receptor (A3AR) antagonist activity. The current study aimed to investigate the anti-obesity effects of LJ-4378 and its underlying molecular mechanisms.
Methods
Immortalized brown adipocytes were used for in vitro analysis. A high-fat diet (HFD)-induced obesity and cell death-inducing DFFA-like effector A reporter mouse models were used for in vivo experiments. The effects of LJ-4378 on lipolysis and mitochondrial metabolism were evaluated using immunoblotting, mitochondrial staining, and oxygen consumption rate analyses. The in vivo anti-obesity effects of LJ-4378 were evaluated using indirect calorimetry, body composition analyses, glucose tolerance tests, and histochemical analyses.
Results
In vitro LJ-4378 treatment increased the levels of brown adipocyte markers and mitochondrial proteins, including uncoupling protein 1. The effects of LJ-4378 on lipolysis of adipocytes were more potent than those of the A2AAR agonist or A3AR antagonist. In vivo, LJ-4378 treatment increased energy expenditure by 17.0% (P value < 0.0001) compared to vehicle controls. LJ-4378 (1 mg/kg, i.p.) treatment for 10 days reduced body weight and fat content by 8.24% (P value < 0.0001) and 24.2% (P value = 0.0044), respectively, and improved glucose tolerance in the HFD-fed mice. LJ-4378 increased the expression levels of brown adipocyte markers and mitochondrial proteins in interscapular brown and inguinal white adipose tissue.
Conclusion
These findings support the in vivo anti-obesity effects of LJ-4378, and suggest a novel therapeutic approach to combat obesity and related metabolic diseases.
Similar content being viewed by others
Introduction
Obesity is defined as the excess accumulation of fat, which is a major risk factor for various metabolic diseases, such as type 2 diabetes [1]. Adipose tissue can be categorized into brown adipose tissue (BAT) and white adipose tissue (WAT) [2]. While BAT contains numerous mitochondria and is responsible for non-shivering thermogenesis, WAT specializes in fat storage and mobilization [3]. Thus, the activation and recruitment of BAT is a strategy to increase energy expenditure, which can ultimately be developed as a potential anti-obesity target [4].
The pharmacological stimulation of adenosine receptor signaling has received significant attention as a potential target for improving metabolic health [5]. Adenosine receptors can be categorized into four types: A1, A2A, A2B, and A3 [6]. The A1 adenosine receptor (A1AR) and A3 adenosine receptor (A3AR) coupled to Gi proteins inhibit the adenylate cyclase (AC)-mediated cAMP production and cAMP-dependent protein kinase (PKA) signaling. In contrast, the A2A adenosine receptor (A2AAR) and A2B adenosine receptor (A2BAR) are coupled to Gs proteins, facilitating AC activity [7].
Among adenosine receptors, A2AAR is more abundantly expressed in BAT than in WAT [8]. A2AAR signaling is necessary for the complete physiological function of BAT, and its anti-obesity effects have been supported by several studies [9]. Although A3AR is expressed in WAT [8], its regulatory roles in adipocyte metabolism are not yet fully understood.
In this study, we investigated the anti-obesity effects of LJ-4378, a dual-acting ligand with A2AAR agonist and A3AR antagonist activities [10], using a high-fat diet (HFD)-induced obesity mouse model. The in vitro effects of LJ-4378 on lipolysis and mitochondrial metabolism were evaluated using immortalized brown adipocytes and compared to those of the A2AAR agonist and A3AR antagonist.
Methods
Cell culture
Brown adipocytes were differentiated from immortalized preadipocytes obtained from interscapular BAT of mice as previously described [11]. Cells were cultured in Dulbecco’s modified Eagle’s medium (Welgene, LM001-07, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS, Gibco, 16000044, Waltham, MA, USA) and 1% Penicillin Streptomycin (Welgene, LS202-02, Waltham, MA, USA) at 37 °C in a humidified atmosphere with 5% CO2. When the cells reached about 90% confluency, the cells were exposed to a differentiation medium supplemented with 2.5 mM isobutyl methylxanthine (IBMX, Cayman, I5879, Ann Arbor, MI, USA), 0.125 mM indomethacin (Cayman, 70270, Ann Arbor, MI, USA), 1 μM dexamethasone (Cayman,11015, Ann Arbor, MI, USA), 1 μg/mL insulin (Sigma, I9278, St. Louis, MO, USA), and 1 nM triiodothyronine (T3, Cayman, 6028, Arbor, MI, USA) for 3 days for adipogenic differentiation. Then the cells were maintained in a growth media with 1 μg/mL insulin and 1 nM T3 (triiodothyronine) for 3 days. Intracellular cAMP levels were measured using the Direct cAMP ELISA Kit (Enzo Life Science, ADI-901-066A, New York, NY, USA) in accordance with the manufacturer’s protocol.
3T3-L1 cells obtained from ATCC (Manassas, VA, USA) were cultured in growth medium (Dulbecco’s Modified Eagle’s Medium (Welgene, LM001-07, St. Louis, MO, USA) supplemented with 10% FBS and 1% Penicillin Streptomycin)) at 37 °C in a humidified atmosphere with 5% CO2 [11]. Cells were then exposed to adipogenic differentiation medium (growth medium supplemented with 0.125 mM indomethacin (Cayman, 70270, Ann Arbor, MI, USA), 2.5 mM isobutyl methylxanthine (IBMX, Cayman, I5879, Ann Arbor, MI, USA), 1 µM dexamethasone (Cayman,11015, Ann Arbor, MI, USA), 1 μg/mL insulin (Sigma, I9278, St. Louis, MO, USA), and 1 nM triiodothyronine (T3, Cayman, 6028, Arbor, MI, USA)) for 3 days. For maintenance of differentiation, cells were exposed to a maintenance medium (growth medium supplemented with 1 µg/mL insulin and 1 nM triiodothyronine) for 3 days. For siRNA knockdown, fully differentiated 3T3L1 adipocytes were transfected with Negative Control siRNA (Bioneer, SN-1013, Daejeon, Republic of Korea) or A2AAR siRNA (Bioneer, 1211, Daejeon, Republic of Korea) using INTERFERin (Polyplus, 409-10, Florida, NY, USA) according to the manufacturer’s protocol. For in vitro experiments, cells were randomly assigned to each experimental group.
The LJ-4378, LJ-4433, and LJ-529 were synthesized as described previously [10, 12, 13]. CGS21680 (Cayman, 124431-80-7, Ann Arbor, MI, USA) was used for experiments.
Oxygen consumption rate (OCR)
XFp Analyzers were used to measure oxygen consumption rate (OCR). Cells were incubated in XF DMEM base medium (Agilent, 103575-100, pH 7.4, Cedar Creek, Texas, USA) supplemented with 4 mM L-glutamine (Sigma, G8540, St. Louis, MO, USA) and 25 mM D-glucose (Sigma, G7021, St. Louis, MO, USA) at 37 °C for the measurement. XFp Cell Mito Stress Test Kit (Agilent, 103010-100, Cedar Creek, Texas, USA) was sequentially prepared with the following optimal final concentrations: 2.5 μM oligomycin, 0.5 μM FCCP, and 0.5 μM rotenone/antimycin A, and basal, maximal, proton leak and were calculated previously described [14].
Immunocytochemistry
Cells were exposed to MitoTracker™ Red CMXRos (1:3000, Invitrogen, M7512, Waltham, MA, USA) for 15 min at 37 °C for mitochondria staining, then fixed with 4% paraformaldehyde (PFA, Sigma, 158127, St. Louis, MO, USA) as previously described [15]. Images were obtained using LSM800 confocal microscope (Zeiss, Germany) and analyzed with Zen software (version 3.0).
Cytotoxicity assay
EZ-CYTOX (DoGEN, EZ-3000, Seoul, Republic of Korea) was used to evaluate the cell viability, according to the manufacturer’s instructions. Absorbance was measured at 450 nm using a microplate reader (Thermo MULTISKAN GO, 8816-2015, Waltham, MA, USA).
Analysis of glycerol and free fatty acid (FFA) assay
Glycerol and free fatty acid (FFA) levels in media were measured using glycerol reagent (Sigma-Aldrich, F6428, St. Louis, MO, USA) and NEFA reagents (WAKO, 436-91693, Osaka, Japan) following the manufacturer’s product protocol as previously described [16].
Animals
Animal experiments were performed following approved protocols by Institutional Animal Care and Use Committees of Seoul National University (SNU-201107-1, SNU-201221-3). C57BL/6 male mice and CIDEA reporter mice [17] were used (8-week-old, male). Mice were housed at 12 h-light/12 h-dark cycle condition with free access to a normal chow diet (NCD, Purina Lab, 38057, protein: 24.52% calories, carbohydrates: 63.07% calories, fat: 12.41% calories, Seongnam, Republic of Korea) and water at 22 ± 1 °C. For the diet-induced obesity model, 8-week-old male mice were fed a HFD (Research Diets, D12492, protein: 20% kcal, carbohydrate: 20% kcal, fat: 60% kcal, New Brunswick, NJ, USA) for 8 weeks. Mice were treated either with LJ-4378 (1 mg kg−1 day−1) or vehicle intraperitoneally for 10 days [8]. LJ-4378 was dissolved in dimethylsulfoxide (DMSO) and diluted in sterile 0.9% saline (0.2% DMSO as the final concentration).
Indirect calorimetry was performed to measure expenditure (EE), VO2, VCO2, activity and food intake using PhenoMaster (TSE Systems, Bad Homburg, Germany). Body composition was measured by nuclear magnetic resonance scanning EchoMRI-700 (Echo Medical Systems, India).
Mice were randomly assigned to experimental groups, and experimental groups of mice were not blinded.
Western blot analysis and quantitative PCR
Western blot analysis and qPCR were conducted, as described previously [17]. Primers used for the qPCR analyses are listed in Supplementary Table S1. Primary antibodies used for western blot are summarized in Supplementary Table S2. Anti-rabbit horseradish peroxidase antibodies (1:3000, Thermo Fisher, 31460, Waltham, MA, USA) and anti-mouse horseradish peroxidase antibodies (1:3000, Jackson, 115-035-174, USA) were used for secondary antibodies. All antibodies were diluted in blocking buffer (5% skim milk or 5% bovine serum albumin in TBST). Western blot images were acquired using the Fusion Solo chemiluminescence imaging system (Vilber Lourmat, France) and analyzed with EvolutionCapt software (version 17.03). Images of the whole western blot membrane are provided in Supplementary Data. NIH ImageJ software was used for the quantification.
Histology
Adipose tissues were fixed using 10% formalin (Sigma, St. Louis, MO, USA) and then embedded in paraffin blocks. The paraffin sections were stained with hematoxylin/eosin (H&E) (BBC biochemical, McKinney, TX, USA) as described previously [14].
Glucose tolerance test
For the glucose tolerance test, mice were intraperitoneally injected with 20% D-Glucose (1 g kg−1, Sigma-Aldrich, G7021, St. Louis, MO, USA) as described previously [18]. A glucose meter (Gluco Dr. Top, allmedicus, AGM-4100, Anyang, Republic of Korea) was used to measure glucose concentration.
TTC analysis
Ex vivo electron transport activity related to mitochondrial oxidative phosphorylation was evaluated by monitoring the reduction of 0.1% triphenyltetrazolium chloride (TTC, Sigma, T8877, St. Louis, MO, USA) as described previously [16].
Bioluminescence and fluorescence imaging
In vivo bioluminescence was detected as previously described using an optical imaging device (Ami-X, Spectral Instruments Imaging) [17]. To detect in vivo bioluminescence signal, mice were i.p. injected with D-luciferin (150 mg kg−1, Goldbio, St. Louis, MO, USA). Aura Software (Spectral Instruments Imaging, Version 2.2.1.1) was used for quantification. Also, ex vivo imaging, tissues were collected from the CIDEA reporter mice treated with D-luciferin (150 mg kg−1, Goldbio, St. Louis, MO, USA). During imaging, the isolated tissues were maintained in 12 well plates containing D-luciferin (300 μg/ml).
Statistical analysis
Sample sizes were determined based on reproducibility and statistical significance. Prism 7 software (GraphPad Software, USA) was used for statistical analysis. Data were shown as mean ± SEM. An unpaired t-test was used between two groups to measure statistical significance. Data were normally distributed with equal variances between sample groups. No data were excluded from in vitro and in vivo experiments.
Results
LJ-4378 treatment upregulates lipolysis and mitochondrial content in a dose-dependent manner in vitro
LJ-4378 is a dual-acting ligand with A2AAR agonist and A3AR antagonist activities (Fig. 1a) [10]. The cytotoxicity assay of LJ-4378 indicated that concentrations below 100 μM did not affect the brown adipocyte viability (Fig. 1b).
a The chemical structure of LJ-4378; (2R,3S,4R)-2-(6-Amino-2-(hex-1-ynyl)-9H-purin-9-yl)- tetrahydrothiophene-3,4-diol. b Cytotoxicity effects of LJ-4378 on brown adipocytes (BAs) at indicated concentration (24 h treatment, n = 5, means ± SEM, ****p < 0.0001). c Immunoblot analysis of hormone-sensitive lipase (HSL) and phosphorylated HSL (p-HSL, serine 660) of BAs treated with LJ-4378 for 4 h (n = 5, means ± SEM, *p < 0.05, ***p < 0.001, ****p < 0.0001). d Free fatty acid (FFA) levels in conditioned media of BAs treated with 0.1 μM LJ-4378 for 24 h (n = 5, means ± SEM, ****p < 0.0001). e Immunoblot analysis of BAs treated with LJ-4378 at indicated concentration for 48 h (n = 5, means ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
As A2AAR activates PKA signaling in adipocytes, the lipolytic effects of LJ-4378 were examined in brown adipocytes differentiated from immortalized brown pre-adipocytes. Immunoblotting analysis showed that LJ-4378 upregulated the protein levels of phosphorylated hormone-sensitive lipase (P-HSL) at serine 660 in a dose-dependent manner (Fig. 1c). Consistently, FFA levels were also increased by LJ-4378 (Fig. 1d: 2.29 ± 0.05 folds increase).
In addition, the levels of mitochondrial proteins involved in oxidative metabolism increased with an increase in the dose of LJ-4378 (Fig. 1e). Based on these dose-response assessments, a concentration of 0.1 μM was selected for all subsequent experiments.
Lipolytic effects of LJ-4378 are more potent than those of the A2AAR agonist or A3AR antagonist
Quantitative polymerase chain reaction (qPCR) analyses were performed to determine the adenosine receptor expression levels in brown adipocytes differentiated from immortalized brown pre-adipocytes. A2AAR showed the highest expression, and A3AR showed the second highest expression among all the adenosine receptors examined (Fig. 2a). The lipolytic effect of LJ-4378 was compared to that of the A2AAR agonist (CGS21680) and A3AR antagonist (LJ-4433). In both the glycerol and FFA assays, LJ-4378 treatment exhibited the greatest increase compared to CGS21680 and LJ-4433 (Fig. 2b, c). Immunoblotting analysis further confirmed the upregulation of the expression levels of PKA downstream proteins, including phosphorylated cAMP response element-binding protein (P-CREB) and P-HSL (Fig. 2d). Consistently, LJ-4378 increased intracellular cAMP levels in BAs (Supplemental Fig. S1). Moreover, immunoblot analysis indicated that A3AR agonist (LJ-529) treatment reduces phosphorylation levels of PKA downstream proteins (Supplementary Fig. S2).
a qPCR analysis of expression levels of Adora1 (A1), Adora2a (A2A), Adora2b (A2B), and Adora3 (A3) adenosine receptors of BAs (n = 5, means ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001). b, c Glycerol and FFA levels in conditioned media of BAs treated with LJ-4378 (0.1 μM), A2A agonist (CGS21680, 0.1 μM), or A3 antagonist (LJ-4433, 0.1 μM) for 24 h (n = 5, means ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). d Immunoblot analysis of BAs treated with LJ-4378 (0.1 μM), A2A agonist (CGS21680, 0.1 μM), or A3 antagonist (LJ-4433, 0.1 μM) for 24 h. (n = 5, means ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Next, we examined the effects of A2AAR knockdown on LJ-4378 induced lipolysis. A2AAR siRNA achieved nearly 70% knockdown of the A2AAR expression in 3T3L1 cells (Supplementary Fig. S3). We confirmed that LJ-4378 treatment increased phosphorylated hormone-sensitive lipase (P-HSL) levels in 3T3L1 cells. A2AAR knockdown significantly suppressed LJ-4378-induced phosphorylation of HSL (Supplementary Fig. S3).
LJ-4378 increases mitochondrial content and metabolism in brown adipocytes
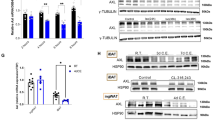
Next, the effects of LJ-4378 on the mitochondrial content and metabolic activity of brown adipocytes were evaluated. The expression levels of the brown adipocyte marker, uncoupling protein 1 (UCP1), and mitochondrial protein, cytochrome c oxidase subunit 4I1 (COXIV), were significantly increased by 2.67 ± 0.38 folds and 2.29 ± 0.11 folds, respectively, after LJ-4378 treatment (Fig. 3a). MitoTracker staining also demonstrated that LJ-4378 increased mitochondrial membrane potential by 2.12 ± 0.06 folds in brown adipocytes (Fig. 3b). The OCRs of basal, maximal, and ATP production-related respiration were increased by LJ-4378 (Fig. 3c). Furthermore, we compared the effects of LJ-4378 on the mitochondrial content with those of CGS21680 and LJ-4433. While both CGS21680 and LJ-4433 increased UCP1, COXIV, and MCAD levels, greater effects were observed in LJ-4378 (Fig. 3d).
a Immunoblot analysis of BAs treated with 0.1 μM LJ-4378 for 24 h (n = 5, means ± SEM, ***p < 0.001, ****p < 0.0001). b Representative images of MitoTracker staining of BAs treated with vehicle (CTL) and 0.1 μM LJ-4378 (n = 5, size bar = 20 μm). c OCR measurement of BAs treated with 0.1 μM LJ-4378 for 24 h (n = 3, means ± SEM, *p < 0.05, **p < 0.01). d Immunoblot analysis of BAs treated with LJ-4378 (0.1 μM), A2A agonist (CGS21680, 0.1 μM), or A3 antagonist (LJ-4433, 0.1 μM) for 24 h (n = 5, means ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
LJ-4378 increases the mitochondrial activity in adipose tissues and energy expenditure in vivo
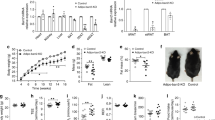
The effects of LJ-4378 were investigated in vivo. In situ staining with triphenyltetrazolium chloride (TTC), a redox indicator, demonstrated that LJ-4378 upregulated mitochondrial activity in all adipose tissue depots (Fig. 4a). Indirect calorimetric analysis further indicated that LJ-4378 treatment increased oxygen consumption by 17.8%, thereby increasing energy expenditure by 17.0%, while activity and food intake were unaffected (Fig. 4b).
Mice were treated with vehicle (CTL), LJ-4378 (LJ, i.p., 1 mg kg−1/day) for 10 days. a 2,3,5-Triphenyltetrazolium chloride (TTC) staining of BAT, iWAT, and gWAT (n = 5, means ± SEM, **p < 0.01, ***p < 0.001, ****p < 0.0001). b Indirect calorimetry analysis of EE (energy expenditure), VO2 (rate of oxygen consumption), VCO2 (rate of carbon dioxide production), RER (respiratory exchange ratio), Activity Counts, food consumption (FEED) (n = 5, means ± SEM, **p < 0.01).
LJ-4378 treatment increases the browning of adipose tissues in vivo
The effects of LJ-4378 on adipose tissue browning were examined in the cell death-inducing DFFA-like effector A (CIDEA) reporter mice. CIDEA is a marker of brown adipocytes, and this reporter system enables non-invasive monitoring of CIDEA expression based on bioluminescence signals. LJ-4378 significantly increased the bioluminescence intensities of BAT and inguinal white adipose tissue (iWAT) (Fig. 5a, b). In particular, there was a significant increase in iWAT compared with CGS21680 and LJ-4433. In line with the in vivo bioluminescence results, LJ-4378 treatment resulted in the most significant increase in the ex vivo luminescence signals of BAT and iWAT (Fig. 5c).
Mice were treated with vehicle (CTL), LJ-4378 (LJ, 1 mg kg−1/day), A2A agonist (CGS21680, 1 mg kg−1/day), or A3 antagonist (LJ-4433, 1 mg kg−1/day) for 10 days. a, b Representative bioluminescence of dorsal and lateral positioning (n = 3, means ± SEM, **p < 0.01, ****p < 0.0001). c A representative ex vivo fluorescence image of BAT and iWAT and quantification of fluorescence intensity (n = 3, means ± SEM, **p < 0.01, ***p < 0.001, ****p < 0.0001). d qPCR analysis of Ucp1 and Cidea mRNA expression level of brown adipose tissue (BAT) (n = 3, means ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001).
The mRNA levels of brown adipocyte markers were also examined and it was demonstrated that LJ-4378 resulted in the greatest increase in gene expression related to browning, including Ucp1 and Cidea in BAT, among the adenosine receptor modulators (Fig. 5d).
LJ-4378 protects mice against diet-induced obesity
We then examined whether the lipolysis and browning effects of LJ-4378 would have an in vivo anti-obesity effect using an HFD-induced obesity mouse model. LJ-4378-treated HFD-fed mice showed 8.24% and 24.18% reduction in body weight and adiposity, respectively (Fig. 6a, b). Moreover, the iWAT and gonadal white adipose tissue (gWAT) weights were significantly decreased by LJ-4378 treatment in HFD-fed mice (Fig. 6c). LJ-4378 improved glucose tolerance in the HFD-fed mice (Fig. 6d). In addition, the reduced size of adipocytes in iWAT and gWAT was observed in H&E-stained sections (Fig. 6e, f and Supplementary Fig. S4).
Normal chow diet (NCD) and high-fat diet (HFD)-fed mice were treated with LJ-4378 (LJ, 1 mg kg−1/day) or vehicle controls (CTL) for 10 days. a Body weight changes (n = 6). b Body composition of fat percentage and lean percentage (n = 6, means ± SEM, **p < 0.01). c Tissue weight (mg) per g body weight of BAT, iWAT, gWAT (n = 6, means ± SEM, *p < 0.05, **p < 0.01). d Glucose tolerance test and area under the curve (AUC) (n = 5, means ± SEM, *p < 0.05). e, f Hematoxylin and eosin (H/E) staining of paraffin sections of BAT, iWAT, and gWAT (size bar = 10 μm). g Immunoblot analysis in BAT (n = 6, means ± SEM, **p < 0.01, ****p < 0.0001). h Immunoblot analysis in iWAT. Tubulin was the loading control. (n = 6, means ± SEM, *p < 0.05, ***p < 0.001, ****p < 0.0001).
Next, we examined the expression levels of mitochondrial proteins and PKA downstream markers in adipose tissue. LJ-4378 upregulated UCP1, COXIV, P-CREB, and P-HSL in BAT and iWAT of HFD-fed mice (Fig. 6g, h). However, LJ-4378 treatment did not significant effects on mitochondrial protein expression in muscle (Supplementary Fig. S5).
qPCR analysis of expression levels of genes involved in inflammatory responses demonstrated that LJ4378 treatment increased the anti-inflammatory markers (Arg1 in iWAT and Il10 in gWAT). By contrast, a pro-inflammatory marker Tnfa was reduced in both iWAT and gWAT (Supplementary Fig. S6).
Discussion
Extracellular adenosine is a potent endogenous signaling molecule that regulates multiple physiological and pathological events via four types of adenosine receptors [9]. Studies using transgenic mice with altered adenosine receptor expression further support that adenosine exerts multiple effects on energy metabolism [19, 20]. Thus, targeting adenosine receptors may have translational potential for the treatment of metabolic diseases. This study investigated the effects of a dual ligand with A2AAR agonist and A3AR antagonist activities on adipocyte metabolism.
Previous studies demonstrated that A2AAR agonist treatment has anti-obesity effects partly via the activation of BAT thermogenesis and energy expenditure [8] and protected against hypertensive cardiac remodeling by facilitating the secretion of BAT-derived fibroblast growth factor 21, so-called “batokine” [21]; however, its crosstalk or synergistic effects with the activation of other adenosine receptors on adipocyte metabolism have not been fully investigated. While the stimulation of Gαs-coupled A2AAR activates AC and cAMP–PKA signaling pathways, the activation of Gαi-coupled A3AR inhibits AC, opposing the effects of A2AAR signaling. Thus, we hypothesized that the dual ligand with A2AAR agonist and A3AR antagonist activities could synergistically activate PKA-dependent lipolysis and thermogenesis in adipocytes.
This study demonstrated that LJ-4378 treatment increased the mitochondrial content and activity in adipocytes, and its effects were more potent than those of the A2AAR agonist or A3AR antagonist. In vivo, LJ-4378 treatment increased energy expenditure and the expression of the brown adipocyte marker CIDEA, which was determined by non-invasive imaging of bioluminescence signaling in CIDEA reporter mice. In vivo, LJ-4378 treatment reduced body weight and fat content and improved glucose tolerance in HFD-fed mice, indicating its anti-obesity effects.
qPCR analysis in the current study indicated that A2AAR and A3AR are the major subtypes of adenosine receptors expressed in adipocytes. In contrast, the expression levels of A2BAR in adipocytes were lower than those of other adenosine receptors [22]. A2BAR is expressed mainly in the muscle and BAT, and the activation of A2BAR exerts anti-aging and anti-obesity effects [22]. Interestingly, A2BAR has a permissive role in A2A-mediated lipolysis by forming heterodimers of the two receptors [22]. In contrast, A1 receptors, which are coupled to Gαi, inhibit AC activity and are highly expressed in human white adipocytes [9]. A1ARs have been characterized as lipolysis inhibitors [9] that can promote insulin sensitivity in adipose tissue by reducing circulating FFA and triglyceride levels [23]. Moreover, A1AR signaling promotes lipogenesis and modulates inflammation, as shown in a A1AR knockout mouse study [24]. Further investigation is required to understand the contribution of individual and interactive adenosine receptor signaling to the development of novel therapeutics that target adenosine signaling to efficiently activate lipid catabolism and restore insulin sensitivity in adipocytes.
In addition to its effects on lipolysis, A2AAR activation exerted anti-inflammatory effects in a mouse model of diet-induced obesity [25]. In our previous study, LJ-4378 demonstrated in vivo anti-inflammatory effects in a rodent model of carrageenan-induced paw edema [10]. Although we focused on the thermogenic and lipolytic effects of the dual ligands, further studies are warranted to determine the effect of LJ-4378 on the polarization of adipose tissue macrophages and HFD-induced inflammation.
Other Gs-coupled receptors have been investigated for their role in the activation of BAT thermogenesis. Although β3-adrenergic receptor-mediated signaling pathways are canonical pathways that activate the thermogenic function of BAT, the effects of β3-selective agonists in humans were unexpectedly low in clinical trials. Adenosine treatment inhibits isoproterenol-mediated lipid metabolism in brown adipocytes of hamsters, which contradicts the results obtained from mouse studies [26, 27]. However, these discrepancies might be due to the species-specific expression and activity of adenosine receptor isotypes [8]. In this regard, understanding the species-specificity of receptor expression and activity is a prerequisite for translational approaches.
The effects of adenosine signaling in a broad range of tissues may limit the clinical application of adenosine receptor ligands. For example. A2AARs play important roles in the pathogenesis of hepatic fibrosis and cirrhosis [28, 29]. The limitation of current study is that the role of non-adipose tissues in mediating the in vivo effects of LJ-4378 cannot be ruled out until pharmacokinetic-pharmacodynamic studies and tissue-specific knockout studies are completed. Furthermore, tissue- or cell-type-specific targeting of pharmacological modulators of adenosine receptor signaling is necessary to develop therapeutic tools without potential adverse effects.
Conclusions
In summary, our data demonstrate that the simultaneous regulation of adenosine A2AAR and A3AR activity has in vivo anti-obesity effects. These findings may aid in the development of a novel therapeutic approach for combating obesity and related metabolic diseases.
Data availability
All data generated or analyzed during this study are included in this published paper and its supplementary information files.
References
Schetz M, De Jong A, Deane AM, Druml W, Hemelaar P, Pelosi P, et al. Obesity in the critically ill: a narrative review. Intens Care Med. 2019;45:757–69.
Becher T, Palanisamy S, Kramer DJ, Eljalby M, Marx SJ, Wibmer AG, et al. Brown adipose tissue is associated with cardiometabolic health. Nat Med. 2021;27:58–65.
Zhang F, Hao G, Shao M, Nham K, An Y, Wang Q, et al. An Adipose Tissue Atlas: An Image-Guided Identification of Human-like BAT and Beige Depots in Rodents. Cell Metab. 2018;27:252–62.e3
Carlsen H, Haugen F, Zadelaar S, Kleemann R, Kooistra T, Drevon CA, et al. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr. 2009;4:215–22.
Im H, Park JH, Im S, Han J, Kim K, Lee YH. Regulatory roles of G-protein coupled receptors in adipose tissue metabolism and their therapeutic potential. Arch Pharm Res. 2021;44:133–45.
Csóka B, Koscsó B, Töro G, Kókai E, Virág L, Németh ZH, et al. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes. 2014;63:850–66.
Tozzi M, Novak I. Purinergic Receptors in Adipose Tissue As Potential Targets in Metabolic Disorders. Front Pharmacol. 2017;8:878.
Gnad T, Scheibler S, von Kügelgen I, Scheele C, Kilić A, Glöde A, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. 2014;516:395–9.
Antonioli L, Blandizzi C, Csóka B, Pacher P, Haskó G. Adenosine signalling in diabetes mellitus–pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2015;11:228–41.
Hou X, Majik MS, Kim K, Pyee Y, Lee Y, Alexander V, et al. Structure-activity relationships of truncated C2- or C8-substituted adenosine derivatives as dual acting A2A and A3 adenosine receptor ligands. J Med Chem. 2012;55:342–56.
Kim SN, Kwon HJ, Akindehin S, Jeong HW, Lee YH. Effects of Epigallocatechin-3-Gallate on Autophagic Lipolysis in Adipocytes. Nutrients. 2017;9:680.
Jeong LS, Choe SA, Gunaga P, Kim HO, Lee HW, Lee SK, et al. Discovery of a new nucleoside template for human A3 adenosine receptor ligands: D-4’-thioadenosine derivatives without 4’-hydroxymethyl group as highly potent and selective antagonists. J Med Chem. 2007;50:3159–62.
Chung H, Jung JY, Cho SD, Hong KA, Kim HJ, Shin DH, et al. The antitumor effect of LJ-529, a novel agonist to A3 adenosine receptor, in both estrogen receptor-positive and estrogen receptor-negative human breast cancers. Mol Cancer Ther. 2006;5:685–92.
Cho YK, Son Y, Saha A, Kim D, Choi C, Kim M, et al. STK3/STK4 signalling in adipocytes regulates mitophagy and energy expenditure. Nat Metab. 2021;3:428–41.
Akindehin S, Jung YS, Kim SN, Son YH, Lee I, Seong JK, et al. Myricetin Exerts Anti-Obesity Effects through Upregulation of SIRT3 in Adipose Tissue. Nutrients. 2018;10:1962.
Choi C, Son Y, Kim J, Cho YK, Saha A, Kim M, et al. TM4SF5 Knockout Protects Mice From Diet-Induced Obesity Partly by Regulating Autophagy in Adipose Tissue. Diabetes. 2021;70:2000–13.
Son Y, Choi C, Song C, Im H, Cho YK, Son JS, et al. Development of CIDEA reporter mouse model and its application for screening thermogenic drugs. Sci Rep. 2021;11:18429.
Kwon HJ, Saha A, Ahn SY, Cho YK, Son Y, Kim M, et al. Polymethoxyselenoflavones exert anti-obesity effects through activation of lipolysis and brown adipocyte metabolism. Int J Obes (Lond). 2021;45:122–9.
Xiao C, Liu N, Jacobson KA, Gavrilova O, Reitman ML. Physiology and effects of nucleosides in mice lacking all four adenosine receptors. PLoS Biol. 2019;17:e3000161.
Yaar R, Jones MR, Chen JF, Ravid K. Animal models for the study of adenosine receptor function. J Cell Physiol. 2005;202:9–20.
Ruan CC, Kong LR, Chen XH, Ma Y, Pan XX, Zhang ZB, et al. A(2A) Receptor Activation Attenuates Hypertensive Cardiac Remodeling via Promoting Brown Adipose Tissue-Derived FGF21. Cell Metab. 2018;28:476–89.e5
Gnad T, Navarro G, Lahesmaa M, Reverte-Salisa L, Copperi F, Cordomi A, et al. Adenosine/A2B Receptor Signaling Ameliorates the Effects of Aging and Counteracts Obesity. Cell Metab. 2020;32:56–70.e7
Meriño M, Briones L, Palma V, Herlitz K, Escudero C. Role of adenosine receptors in the adipocyte-macrophage interaction during obesity. Endocrinol Diabetes Nutr. 2017;64:317–27.
Johansson SM, Lindgren E, Yang JN, Herling AW, Fredholm BB. Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue-interactions with insulin. Eur J Pharmacol. 2008;597:92–101.
Pei Y, Li H, Cai Y, Zhou J, Luo X, Ma L, et al. Regulation of adipose tissue inflammation by adenosine 2A receptor in obese mice. J Endocrinol. 2018;239:365–76.
Szillat D, Bukowiecki LJ. Control of brown adipose tissue lipolysis and respiration by adenosine. Am J Physiol. 1983;245:E555–9.
Schimmel RJ, McCarthy L. Role of adenosine as an endogenous regulator of respiration in hamster brown adipocytes. Am J Physiol. 1984;246:C301–7.
Fausther M. Extracellular adenosine: a critical signal in liver fibrosis. Am J Physiol-Gastrointest Liver Physiol. 2018;315:G12–G9.
Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, et al. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol. 2006;148:1144–55.
Acknowledgements
The authors acknowledge Heeseong Kim for technical support. This research was supported by the National Research Foundation of Korea (NRF) grants (2019R1C1C1002014 and 2018R1A5A2024425 to YHL) funded by the Korean government (Ministry of Science and ICT).
Author information
Authors and Affiliations
Contributions
YHL and LSJ conceived and designed the study. KK, HI, YS, MK, and SKT conducted and analyzed the experiments. YHL, KK, and HI wrote the paper. All authors reviewed the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, K., Im, H., Son, Y. et al. Anti-obesity effects of the dual-active adenosine A2A/A3 receptor-ligand LJ-4378. Int J Obes 46, 2128–2136 (2022). https://doi.org/10.1038/s41366-022-01224-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-022-01224-x