Abstract
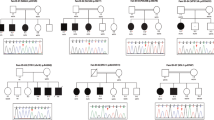
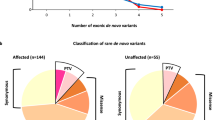
Background: Syndromic obesity (SO) refers to obesity with additional phenotypes, including intellectual disability (ID)/developmental delay (DD), dysmorphic features, or organ-specific abnormalities. SO is rare, has high phenotypic variability, and frequently follows a monogenic pattern of inheritance. However, the genetic etiology of most cases of SO has not been elucidated. Subjects and methods: In this study, we investigated 20 SO patients by whole-exome sequencing (WES) trios to identify causal genetic variants. Results: 4/20 patients had negative results for array comparative genomic hybridization (aCGH) analyses. In the remaining 15 patients, in addition to SNVs and indels, CNVs were also evaluated. Pathogenic/likely pathogenic (P/LP) SNVs/indels were detected in 6/20 patients (involving MED13L, AHDC1, EHMT1, MYT1L, GRIA3, and SETD1A), while two patients carried an inherited VUS. In addition, P/LP CNVs were observed in 3/15 patients (involving SATG2, KIAA0442, and MEIS2). Conclusions: All nine detected P/LP variants involved genes already known to lead to syndromic ID/DD; however, for only two genes (EHMT1 and MYT1L) is the link with obesity well established. This is the first study applying a comprehensive genomic investigation of an SO cohort, showing a high diagnostic yield (~47%). Additionally, our findings suggested that several known ID/DD genes may also predispose individuals to SO.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Data on the genetic variants relevant to this study are openly available in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/). Accession codes are given below: SCV002060423; SCV002060422; SCV002060421; SCV002060420; SCV002060419; SCV002060418; SCV001478246; SCV001432217.
References
D’Angelo CS, Varela MC, De Castro CIE, Otto PA, Perez ABA, Lourenço CM, et al. Chromosomal microarray analysis in the genetic evaluation of 279 patients with syndromic obesity. Mol Cytogenet. 2018;11. https://doi.org/10.1186/s13039-018-0363-7
Geets E, Meuwissen MEC, Van Hul W. Clinical, molecular genetics and therapeutic aspects of syndromic obesity. Clin Genet. 2019;95:23–40. https://doi.org/10.1111/cge.13367
Delrue M-A, Michaud J. Fat chance: genetic syndromes with obesity. Clin Genet. 2004;66:83–93. https://doi.org/10.1111/j.0009-9163.2004.00300.x
Kaur Y, de Souza RJ, Gibson WT, Meyre D. A systematic review of genetic syndromes with obesity. Obes Rev. 2017;18:603–34. https://doi.org/10.1111/obr.12531
Silva M, de Leeuw N, Mann K, Schuring-Blom H, Morgan S, Giardino D, et al. European guidelines for constitutional cytogenomic analysis. Eur J Hum Genet. 2019;27:1–16. https://doi.org/10.1038/s41431-018-0244-x
Maltese PE, Iarossi G, Ziccardi L, Colombo L, Buzzonetti L, Crinò A, et al. A Next Generation Sequencing custom gene panel as first line diagnostic tool for atypical cases of syndromic obesity: Application in a case of Alström syndrome. Eur J Med Genet. 2018;61:79–83. https://doi.org/10.1016/j.ejmg.2017.10.016
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: A jointconsensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24. https://doi.org/10.1038/gim.2015.30
Willemsen MHH, Vulto-van Silfhout ATT, Nillesen WMM, Wissink-Lindhout WMM, van Bokhoven H, Philip N, et al. Update on Kleefstra syndrome. Mol Syndromol. 2011;2:202–12. https://doi.org/10.1159/000335648
Carvalho LML, D’Angelo CS, Mustacchi Z, da Silva IT, Krepischi AC V., Koiffmann CP, et al. A novel MYT1L mutation in a boy with syndromic obesity: Case report and literature review. Obes Res Clin Pract. 2021; https://doi.org/10.1016/j.orcp.2021.01.001
Vuillaume ML, Naudion S, Banneau G, Diene G, Cartault A, Cailley D, et al. New candidate loci identified by array-CGH in a cohort of 100children presenting with syndromic obesity. Am J Med Genet Part A. 2014;164:1965–75. https://doi.org/10.1002/ajmg.a.36587
Emerson E, Robertson J, Baines S, Hatton C. Obesity in British children with and without intellectual disability: cohort study. BMC Public Health. 2016;16:644 https://doi.org/10.1186/s12889-016-3309-1
Hsieh K, Rimmer JH, Heller T. Obesity and associated factors in adults with intellectual disability. J Intellect Disabil Res. 2014;58:851–63. https://doi.org/10.1111/jir.12100
Martins LB, Monteze NM, Calarge C, Ferreira AVM, Teixeira AL. Pathways linking obesity to neuropsychiatric disorders. Nutrition. 2019;66:16–21. https://doi.org/10.1016/j.nut.2019.03.017
Kleinendorst L, Abawi O, van der Voorn B, Jongejan MHTM, Brandsma AE, Visser JA, et al. Identifying underlying medical causes of pediatric obesity: Results of a systematic diagnostic approach in a pediatric obesity center. Buchner DA, editor. PLoS One. 2020;15:e0232990 https://doi.org/10.1371/journal.pone.0232990
Beunders G, van de Kamp J, Vasudevan P, Morton J, Smets K, Kleefstra T, et al. A detailed clinical analysis of 13 patients with AUTS2 syndrome further delineates the phenotypic spectrum and underscores the behavioural phenotype. J Med Genet. 2016;53:523–32. https://doi.org/10.1136/jmedgenet-2015-103601
Louw JJ, Corveleyn A, Jia Y, Hens G, Gewillig M, Devriendt K. MEIS2 involvement in cardiac development, cleft palate, and intellectual disability. Am J Med Genet Part A. 2015;167:1142–6. https://doi.org/10.1002/ajmg.a.36989
Kleinendorst L, Massink M, Cooiman MI, Savas M, van der Baan-Slootweg OH, Roelants RJ, et al. Genetic obesity: next-generationsequencing results of 1230 patients with obesity. J Med Genet. 2018;55:578–86. https://doi.org/10.1136/jmedgenet-2018-105315
Xia F, Bainbridge MN, Tan TY, Wangler MF, Scheuerle AE, Zackai EH, et al. De Novo truncating mutations in AHDC1 in individuals with syndromic expressive language delay, hypotonia, and sleep apnea. Am J Hum Genet. 2014;94:784–9. https://doi.org/10.1016/j.ajhg.2014.04.006
Carvalho LML, Costa SS, Campagnari F, Kaufman A, Bertola DR, Silva IT, et al. Two novel pathogenic variants in MED13L: one familial andone isolated case. J Intellect Disabil Res. 2021;65:1049–1057. https://doi.org/10.1111/jir.12891
Trivisano M, Santarone ME, Micalizzi A, Ferretti A, Dentici ML, Novelli A, et al. GRIA3 missense mutation is cause of an x-linkeddevelopmental and epileptic encephalopathy. Seizure. 2020;82:1–6. https://doi.org/10.1016/j.seizure.2020.08.032
Kummeling J, Stremmelaar DE, Raun N, Reijnders MRF, Willemsen MH, Ruiterkamp-Versteeg M, et al. Characterization of SETD1A haploinsufficiency in humans and Drosophila defines a novel neurodevelopmental syndrome. Mol Psychiatry. 2020; https://doi.org/10.1038/s41380-020-0725-5
Roessler E, Belloni E, Gaudenz K, Vargas F, Scherer S, Tsui L, et al. Mutations in the C-Terminal Domain of Sonic Hedgehog CauseHoloprosencephaly. Hum Mol Genet. 1997;6:1847–53. https://doi.org/10.1093/hmg/6.11.1847
Acknowledgements
We are grateful to the families of patients involved in this study. We also thank the Xia-Gibbs Society (parent organization) and Dr. Richard Gibbs (Baylor College of Medicine - USA) for relevant communication. Additionally, it is important to thank Claudia Castro, Luceleni da Silva, Maraisa de Castro Sebastião, Mariana Barros, Dr. Naila Lourenço and Dr. Mustafa Zogbi for technical support. This study was supported by the São Paulo Research Foundation—FAPESP (2018/08486-3, 2012/50981-5, and 2013/08028-1), National Council for Scientific and Technological Development—CNPq (305806/2019-0) and Coordination for the Improvement of Higher Education Personnel—CAPES (1805008).
Author information
Authors and Affiliations
Contributions
CR and CPK conceived and designed the study. CPK, LMLC, and CSD performed the research subject selection. LMLC performed the NGS data analysis and interpretation of genetic variants under the guidance of CR, AK, and DV. SSC and LDC provided relevant technical support. AALJ made important contributions to the discussion of the results. ITS and MOS contributed to bioinformatics processing (alignment to the reference genome and calling of variants). LMLC was responsible for the initial writing of the manuscript and produced the figures and tables under the guidance of CR and AK. All authors have read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This research is in accordance with ethical standards established in the Declaration of Helsinki (1964), its subsequent revisions, and Resolution 466/2012 of the Brazilian National Health Council. The project was approved by the Research Ethics Committee of the Biosciences Institute of the University of São Paulo (CEP protocol — IBUSP 021/2004). Blood samples of the patients and their parents were collected after informed consent was signed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Carvalho, L.M.L., D’Angelo, C.S., Villela, D. et al. Genetic investigation of syndromic forms of obesity. Int J Obes 46, 1582–1586 (2022). https://doi.org/10.1038/s41366-022-01149-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-022-01149-5
This article is cited by
-
A Comprehensive Review of Syndromic Forms of Obesity: Genetic Etiology, Clinical Features and Molecular Diagnosis
Current Obesity Reports (2024)