Abstract
The new 2019 coronavirus 19 disease (CoVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) poses a serious threat to health systems. As a global health problem, this pandemic poses a huge threat to people and is responsible for significant morbidity and mortality worldwide. On the other hand, obesity has also reached epidemic proportions and poses another challenge to the healthcare system. There is increasing evidence of a strong association between obesity and CoVID-19 disease, but the mechanisms underlying the link between the two remain unclear and the role of obesity also remains to be elucidated. In particular obesity-related low-grade inflammation has been hypothesized as the Achille’s heel that could predispose subjects with obesity to a more severe CoVID-19 compared to subjects with normal weight. Hence, we summarized recent evidence on the role of low-grade inflammation in clinical aspects of CoVID-19 in subjects with obesity in both childhood and adulthood. Further, we provide molecular insights to explain this link.
Similar content being viewed by others
Introduction
The world is currently experiencing the coronavirus 19 disease (CoVID-19) pandemic. In late 2019, the CoVID-19 infection began in Wuhan, Hubei, China. Originally called 2019 nCoV, it was renamed CoVID-19 by the World Health Organization in February 2020 [1].
Several attempts have been made to identify the risk factors that could influence the spread of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and that could worsen the prognosis of hospitalized patients. In this respect, it appears that the new pandemic is complicated by an “old one” that is already well defined, namely obesity. Obesity has almost tripled globally since 1975 and has therefore been designated as a pandemic [2]. In 2016, 39% of adults (more than 1.9 billion people) worldwide were overweight and 13% were obese (more than 650 million people), while in 2018, 40 million children under the age of 5 were overweight or obese [3]. Obesity is a major health concern, mainly because of its side effects in humans and the associated morbidity and mortality rates [4, 5]. In particular, body mass index (BMI) has been reported to have a J-shaped association with overall mortality: the estimated hazard ratio per 5 kg/m2 increase in BMI was 1.21 (1.20–1.22) above 25 kg/m2 [6]. In addition, life expectancy from age 40 years was 4.2 years shorter in men with obesity (BMI ≥ 30.0 kg/m2) and 3.5 years shorter in women with obesity than in individuals with normal weight [6].
In fact, it is highly associated with a number of diseases such as cardiovascular disease (CVD), insulin resistance (IR), and type 2 diabetes mellitus (T2DM) [7]. However, it should be noticed that for a given fat mass, there is a large variability in the risk of developing cardiometabolic diseases [8]. Although the strong predictive power has been attributed to increased visceral fat mass, an analysis of the causes of T2DM and CVD, along with a comparison to rare diseases such as lipodystrophy and studying genetically determined fat distribution in the general population, highlights that an impaired ability to enlarge subcutaneous fat in the lower part of the body is also important for predicting the incidence of these cardiometabolic diseases [8].
In addition, obesity has also been reported to be associated with non-alcoholic fatty liver disease (NAFLD) which is a heterogeneous disease, the advanced stages of which have been reported to be strongly affected by comorbidities such as IR and T2DM [9] and various cancers [10, 11]. These associations are likely related to the features of obesity characterized by impaired energy homeostasis that promotes the development of the metabolic syndrome [12], diseased adipose tissue associated with local inflammation, hypoxia, and cellular stress [13], the altered release of cytokines [14], chronic systemic inflammation [15], IR [16], impaired immune response [17], atherosclerosis, and altered cardiac structure and function associated with a greatly increased risk of CVD [7]. Most of these features have direct or indirect negative effects on the balanced immune surveillance system, resulting in impaired immune response, defective chemotaxis, and deregulated immune cell differentiation [18]. Consequently, it is well documented that obesity is associated with an increased risk as well as a worse course of infectious diseases with increased complications, prolonged hospitalization, and increased critical illness [19,20,–21].
The identification of obesity as an independent risk factor for hospitalizations and deaths from influenza A (H1N1) led to the suspicion that it may have a significant impact on the current CoVID-19 pandemic, and concerns about the impact of obesity on CoVID-19 have been further substantiated by preliminary data from several hospitals [22]. Sixteen thousand seven hundred and forty-nine patients hospitalized with CoVID-19 in the UK were included in the World Health Organization ISARIC clinical characterization protocol [23]. Increasing risks of death have been reported with increasing obesity (BMI > 40 kg/m2 fully adjusted hazard ratio (HR) 1.92, 95% confidence interval (CI) 1.72–2.13), and comorbidities such as delete type 2 diabetes T2DM (with a greater HR for those with recent HbA1c ≥ 58 mmol/mol), severe asthma with recent use of an oral corticosteroid, respiratory disease, chronic heart disease, liver disease, stroke/dementia, other neurological diseases, reduced kidney function (with greater HR for lower estimated glomerular filtration rate), autoimmune diseases (rheumatoid arthritis, lupus or psoriasis) and other immunosuppressive condition [23]. Recently, a large cohort study examined factors associated with CoVID-19-related in-hospital deaths in the linked electronic health records of 17 million adult NHS patients [24]. This found that the risk of death increased with the level of obesity: the fully adjusted HR was 1.27 for a BMI of 30– 34.9 kg/m2 and increased to 2.27 for a BMI ≥ 40 kg/m2 [24]. Finally, recently published statistics from a prospective cohort study indicated that obesity is twice as likely to result in CoVID-19 hospitalization in individuals younger than 60 years [25]. The study, which included 5279 patients with CoVID-19 of whom hospital admission was found in 2741 of them, showed that a BMI > 40 kg/m2 was the second strongest independent predictor of hospitalization after age [25]. One of the most probable links between obesity and CoVID-19 is represented by low-grade inflammation which is characterized by increased pro-inflammatory cytokine secretion from adipose tissue and the infiltration of leukocytes, including macrophages, into the adipose tissue [26]. This inflammation state blunts insulin signaling in adipocytes, causing IR, and giving rise to the development of metabolic disorders such as CVD, T2DM, and hypertension which are well-known comorbidities that adversely affect the outcome of patients with CoVID-19 [26]. Furthermore, a negative correlation of SARS-CoV-2 IgG antibodies have been detected with serum levels of pro-inflammatory and metabolic markers of inflammaging and pulmonary inflammation, such as serum amyloid A protein (SAA), C-reactive protein (CRP), ferritin, and BMI but positively associated with non-esterified fatty acids (NEFA) [27]. Serum CRP has been also found to be positively associated with autoimmune antibodies in obesity [28]. Moreover, SARS-CoV-2 infection induces neutralizing antibodies in all lean but only in a few obese CoVID-19 patients [28].
Although less common, CoVID-19 also affects the pediatric age group [29, 30]. Studies suggest that the incidence of CoVID-19 in children and adolescents may be as high as 5% of confirmed cases, with a slightly higher incidence in males [29]. It is also less severe than in adults [29]. In the United States, hospitalization rates among persons younger than 17 years of age ranged from 0.1 to 0.3/100,000 population in March 2020 [30]. From March 1, 2020–August 14, 2021, COVID-NET identified 49.7 cumulative CoVID-19-associated hospitalizations per 100,000 children and adolescents; in particular, rates were highest among children aged 0–4 years (69.2) and adolescents aged 12–17 years (63.7) while it was lowest among children aged 5–11 years (24.0) [31]. Mortality in children and adolescents has also been shown to be lower compared to adults [32, 33]. In an epidemiological study in China (N = 2135 aged <18 years), only one death was described [32]. A systematic review (N = 2228 aged <16 years) found two deaths [33], one of which was the same as in the study previously mentioned [32].
The effects of pediatric obesity on CoVID-19 have not been adequately studied, and some data are inferences because there have not been many studies on this topic in this age group. The three major risk factors linking obesity to CoVID-19 that have been demonstrated for adults are also present in children and adolescents: chronic subclinical inflammation, impaired immune response, and underlying cardiorespiratory disease [34]. Virtually all comorbidities seen in adults can also be observed in children and adolescents [35], and children with obesity have an inadequate immune response to other infections, such as bacterial pneumonia [36], a common severe complication of CoVID-19 [37]. Studies in animal models show that rats fed a high-fat diet have increased expression of the angiotensin-converting enzyme 2 (ACE2) in the lungs, which is the receptor exploited by the virus to enter cells and this may explain the higher severity of disease in individuals with obesity [38]. Zhang et al. have shown that obesity predisposes to high mortality due to CoVID-19 even in young patients aged 14 years and older [39], and it is hypothesized that the very high prevalence of obesity in young people may shift the age curve of mortality in countries where the prevalence of obesity is higher in this group [40].
The current coincidence of CoVID-19 and obesity pandemics exacerbates the above problems. In this review, we summarize data from recent studies concerning the relationship between obesity and CoVID-19 in adults and children focusing on low-grade inflammation as potential links between these diseases, providing also molecular insights.
Obesity and CoVID-19: human evidence
Adulthood
Advanced age and some risk factors, including obesity, are being studied to clarify the exact impact of these factors on CoVID-19 mortality. The strong relationship between BMI and risk of hospitalization with subsequent need for intensive care units’ (ICUs) access for coronavirus is widely documented in the recent scientific literature. The CoVID-19 pandemic is concurrent with an increase in the prevalence of individuals with overweight/obesity in almost every country in the world [41]. In fact, nearly every country today has a prevalence of overweight individuals and individuals with obesity greater than 20% [2, 42, 43]. Previous viral pandemics, such as the one caused by H1N1 influenza, had already highlighted the link between viral infection and obesity, but, currently, the mechanisms of CoVID-19 virus infection in patients with obesity are not fully elucidated [44, 45].
In the USA, the well-known increased prevalence of obesity and the previous experience regarding the impact of obesity on H1N1 influenza mortality, have highlighted the urgency of increased attention to patients with obesity and CoVID-19, suggesting the more aggressive treatment of such patients compared with lean individuals. In addition, the prevalence of adult obesity and severe obesity in 2017–2018 increased from 2009 and 2010 and is now 42% and 9%, respectively [46]. These observations suggest that the proportion of patients with obesity, severe obesity, and CoVID-19 infections will increase compared with the H1N1 experience and the disease will likely have a more severe course in such patients. These observations also emphasize the need for increased vigilance, priority on detection and testing, and aggressive therapy for patients with obesity and CoVID-19 infections [46]. Obesity and overweight do not seem to have an impact only on the course and the outcome of the disease.
Also, with regard to the mere diagnostic aspect of SARS-CoV-2, the data in the literature suggest, in subjects with obesity, an increased risk of positivity and therefore also of infection and/or symptomatic disease [47,48,–49]. Data analysis of a large national cohort of veterans tested for SARS-CoV-2 also showed that among enrolled patients those with higher BMI were more likely to test positive than those of normal weight [50]. Similar evidence emerged also from the cohort study on a large population of patients that confirmed the presence of a positive association between BMI and risk of CoVID-19 diagnosis, especially for subjects with BMI > 40 kg/m2, (HRs 1.22 for a BMI of 31 kg/m2 and HRs 1.28 for a BMI of 40 kg/m2) [51]. In terms of disease severity, patients with SARS-CoV-2 and obesity are more likely to die from CoVID-19. Patients with obesity require admission to ICUs at higher rates [52] and also have complications not directly related to the respiratory system such as acute renal failure and shock [53]. Early in the pandemic, the studies about mortality and CoVID-19 did not provide data on body weight and height, which are used to estimate adipose tissue mass by calculating the BMI [54]. Later, other studies evidenced that each 1-unit increase in BMI was associated with a 12% increase in the risk of severe CoVID-19, and obesity was associated with a three-fold increase in the risk of severe CoVID-19, compared with lean subject [55]. A French study reported that, among 124 patients admitted to the ICU for CoVID-19, 69% were obese or severely obese [56].
Determining the independent effect of obesity on CoVID-19 severity, requires consideration of associated comorbidities (T2DM, CVD, cancer) which may act as confounders or mediators of this relationship [25, 57, 58]. In a population of 511 CoVID-19 patients (24% with obesity and 14% with severe obesity), enrolled among Military Health System beneficiaries, obesity was strongly correlated with CoVID-19 severity, viral load, and antibody response, suggesting the relationship between obesity and CoVID-19 severity may be mediated by different factors [59]. These findings offer new pathophysiological insights into the relationship between obesity and CoVID-19 severity [49, 59]. Morbidity and mortality from CoVID-19 are further increased in subjects with obesity with visceral adiposity and ectopic fat deposition [60]. Therefore, individuals with obesity are assumed to develop more severe CoVID-19 disease than normal-weight individuals, regardless of gender, age, and existence of associated comorbidities [61,62,–63]. Similarly, in a cohort of 200 hospitalized patients, in USA, with CoVID-19 in a minority-predominant population, severe obesity, increasing age, and male sex were independently associated with higher in-hospital mortality and in general worse in-hospital outcomes [64].
There is not complete agreement among authors regarding whether BMI modifies the risk of mortality or intensive care regardless of age. Lighter et al. suggests that adults with obesity under age 60 are more likely to be hospitalized than lean individuals [57]. Other large epidemiologic studies have found that obesity is associated with increased mortality in only certain age groups, particularly in younger people. Eastment et al. has verified a significantly increased mortality in subjects with third-class obesity, compared to lean subjects, much more evident in the group of younger patients, with the age of less than 65 years [47]. Moreover, Fresan, in a large prospective study, found that third-class obesity is a risk factor for hospitalization and for the development of a serious CoVID-19 disease; although the risk remains similar in different age groups, the excessive risk disappears with aging, suggesting the negative effects of obesity, especially in young people [65]. A large study to investigate associations between BMI and risk of CoVID-19 diagnosis, hospitalization, and death after a CoVID-19 diagnosis, accounting for potential effect modification by age and sex, was recently conducted by Recalde et al. [51]. The association between BMI and death was J-shaped, with a modestly higher risk of death among individuals with undernutrition and a more pronounced increasing risk for BMI ≥ 40 kg/m2 [51]. Similarly, in a large prospective, community-based cohort study, Gao et al. found a J-shaped association between BMI and hospitalization due to CoVID-19 and death, stronger for BMI > 23 kg/m2, in addition to a linear association across the entire range of BMI with hospitalization in ICU [66]. This finding does not appear to be attributable to excessive risks of related diseases and the relative risk due to the increase in BMI is more marked in young people under 40 years of age [66].
Moreover, CoVID-19 severity seems to be associated with abdominal adipose tissue distribution, highlighting the potential pathogenic role of visceral adiposity in acute illness, as suggested by Battisti et al. [67]. Similarly, visceral adiposity was proposed as a marker of worse clinical outcomes, as it is associated with the need of intensive care [68], and CT-based quantification of visceral adipose tissue and upper abdominal circumference may therefore be a simple tool for risk assessment in CoVID-19 patients [69].
Childhood
As concern the pediatric age it is important to underline that, although children are less susceptible to CoVID-19, the pre-existing comorbid condition can predispose to severe disease. A total of 292 children confirmed to have CoVID-19 infection were included in the study [70]. The most common associated disease was obesity (5.1%) [70].
The analysis of the data collected since the beginning of the epidemic novel coronavirus epidemic of 2019 (SARS-CoV-2), showed that elderly subjects, especially those with comorbidities, are at greater risk of developing a critical type of CoVID-19, compared to that of younger subjects [71]. On the contrary, young subjects are believed to have only a mild type of CoVID-19, which is unlikely to cause death and often does not require hospital care. Children affected by CoVID-19 are usually subjected to less aggressive treatment. However, the presence of obesity can modify this aspect. Zhang et al conducted a retrospective study including 53 young patients 13 young patients who died of CoVID-19 and 40 matched survivors [39]. Logistic regression was employed to characterize the risk factors of mortality in young CoVID-19 patients with obesity. The deceased patients manifested higher BMI (odds ratio (OR) 1.354; 95% CI 1.075–1.704; P = 0.010), as compared with that of survivors. These data support that obesity could be a risk factor associated with high mortality in young CoVID-19 patients, whereas aggravated inflammatory response, enhanced cardiac injury, and increased coagulation activity are likely to be the mechanisms contributing to the high mortality [39]. Otherwise, another pediatric study was conducted in 49 CoVID-19 patients (aged between 8 and 18 years) [72]. The weight was normal in 76% and the height was normal in 90% while 3% were malnourished and 9% were affected by obesity. There was no correlation between anthropometric values and susceptibility to childhood CoVID-19 infection or the clinical course [72].
To analyze the demographic and clinical risk factors for severe CoVID-19 in a large metropolitan healthcare system (in Houston, USA) a retrospective epidemiological study was conducted on a population of young people (aged between 18 and 29 years) [73]. This study demonstrated by a multivariable logistic regression that obesity (and other comorbidities) is predictive of severe disease diagnoses within 30 days. Therefore, it is necessary to take into account, even for the young population with comorbidities such as obesity, the significant increase in the risk of serious disease [73].
Nevertheless, data are controversial: in a recent retrospective cohort study, 304 patients (Saudi Arabia) who died after being hospitalized with CoVID-19 were analyzed [74]. The proportion of comorbidities was high in deceased patients who were hospitalized with CoVID-19. A higher age, smoking, and respiratory failure were significant predictors of mortality during the early stay in hospitals but, using Cox Regression, obesity was not a significant predictor of mortality [74].
The mechanisms underlying the association between obesity and CoVID-19 severity remain still to be clarified. Nonetheless, the measurement of anthropometric characteristics for obesity is crucial for a better estimation of the risk of complications in CoVID-19 patients.
Obesity, CoVID-19 and low-grade inflammation: molecular insights
Obesity is considered as both a disease in itself and a condition that increases the likelihood of developing several non-communicable diseases [75]. It was demonstrated that obesity also increases the likelihood of developing serious infectious diseases [76], such as 2009 H1N1 pandemic influenza infection [77, 78].
SARS-CoV-2 enters host cells through the interaction of its spike protein to the cell-surface receptor ACE2 [79]. For the completion of this entry process, the expression on cell surface of the type 2 transmembrane serine protease (TMPRSS2) is required. The pathophysiology of multi-organ failure related to SARS-CoV-2 infection includes: direct virus toxicity; dysregulation of the immune response; endothelial cell damage and thrombo-inflammation; dysregulation of the renin–angiotensin–aldosterone system (RAAS) [79].
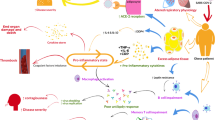
In this context, overweight and obesity which is recognized as a state of low-grade inflammation, a common factor for many chronic diseases, can be a flowery soil for the development of CoVID-19 and for a more severe disease. Hence, we reviewed the possible mechanisms linking obesity to CoVID-19, that appear to interact to each other to induce a worst prognosis in CoVID-19 patients with overweight and obesity (Fig. 1). Indeed, we investigated how the immune response in children with obesity differs from adults and if the presence of obesity may explain the severity associated with CoVID-19.
SARS-CoV-2 pathophysiology includes firstly a cellular tropism versus adipocytes that express a high level of angiotensin-converting enzyme 2 (ACE2), type 2 transmembrane serine protease (TMPRSS2), and transmembrane glycoprotein receptor (CD147). The dysregulation of the immune response by SARS-CoV-2 is characterized by the so-called “cytokine storm” with the increase in interleukin-6 (IL-6), tumor necrosis factor-α (TNFα), interleukin-1β (IL-1β), ferritin and C-reactive protein (CRP) levels, number of macrophages, and the decrease in number of lymphocytes. In turn, low-grade inflammation, the impaired immune system with the increase in IL-6, TNFα, IL-1β, macrophages, leptin, cytotoxic T cells (CD8 + ) and the decrease in adiponectin and regulatory T cells (CD4 + ). Both the diseases impair lipid metabolism with the increase in free fatty acids (FFA) and are potentially trigger of fat embolism syndrome (FES). Hypercoagulopathy, endothelial cell damage and thrombo-inflammation with increase in plasminogen activator inhibitor 1 (PAI-1) and hypoxia-induced factor (HIF)-α levels was described in both obesity and the coronavirus 19 disease (CoVID-19) and are linked to the dysregulation of the immune system. Finally, the dysregulation of the renin–angiotensin–aldosterone system (RAAS) is another common element between the two pathologies. All these mechanisms appear to interact to each other to induce a worst prognosis in CoVID-19 patients with obesity.
Adulthood
Cellular tropism
ACE2 is highly expressed in adipocytes and also in adipocyte-like cells, such as pulmonary lipofibroblasts, which represent the adipocyte equivalent in the lung and are able to dedifferentiate into myofibroblasts that contribute to pulmonary fibrosis [41, 80]. ACE2 expression in adipocytes appears to be stimulated by high-fat diets [81]. Some studies report that drugs used to treat obesity-comorbidities (statins, liraglutide) could induce ACE2 overexpression in adipocytes [82]. These evidences suggest that adipose tissue exhibits a high tropism for the virus infection and highlight the important role of adipose tissue in the pathogenic response to SARS-CoV-2. Furthermore, it was demonstrated that ACE2 was strongly expressed in the pancreatic islets [83]. Considering that patients with obesity and T2DM have a higher expression of ACE2 in adipocytes [84], the adipose tissue becomes a potential target for viral reservoir [80]. In fact, hyperglycemia was considered a worst prognosis factor for CoVID-19, independently from a previous diagnosis of T2DM [85]. It is worth to know that obesity is often characterized by hyperglycemia [86]. In addition, in diet-induced obese male mice ACE2 and TMPRSS2 expression is elevated in the trachea and in the lung compared to females [87]. CD147 is another transmembrane glycoprotein receptor, which expression is high on innate and adaptive memory immune cells. CD147 was shown to act as a SARS-CoV-2 receptor [88] and it was demonstrated that CD147 expression is increased in blood of patients with obesity, correlating positively with BMI [89].
Immune system dysfunction
In obesity, the expansion of white adipose tissue stimulates cytokines and chemotactic factors such as platelet-derived growth factor, monocyte chemoattractant protein-1 (MCP-1), transforming growth factor-β (TGF- β), interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) [90]. These factors recruit and activate endothelial precursor cells, immune cells, and preadipocytes [90]. Thus, both adaptative and innate immunity are impaired in obesity. On the one hand, the dysfunctional adipose tissue triggers a phenotypical switch in macrophage polarization [91], that engages in phagocytosis of dead adipocytes in a configuration termed crown-like structures [92]. On the other hand, there is an increase of neutrophils, CD8+ cytotoxic T cells, natural killer cells, and a decrease in T helper cells CD4+ [93]. The infiltration of macrophages is linked to IR, whereas CD4+ lead to the suppression of IR. Furthermore, obesity-associated inflammation was determined by an increase in the production of leptin, which is pro-inflammatory, through the production of TNF-α and IL-6 and a decrease in adiponectin, which is, through activation of AMP kinase (AMPK), anti-inflammatory and an insulin sensitizer [94]. In addition, leptin promotes the polarization of macrophages toward a pro-inflammatory profile. It changes the T helper cell phenotype by diminishing regulatory T-cells activity and favoring T helper 17 (Th17) polarization [94].
Interestingly, during the H1N1 pandemic it was demonstrated that patients with overweight and obesity had a defective cellular immune response to pandemic influenza A virus and lower production of interferon IFN-γ (IFN-γ), a well-known antiviral protein [78].
It was demonstrated that CoVID-19 is an inflammatory disease, involving all organs and determining organ dysfunction. The replication of the virus is accompanied by a rapid activation of mononuclear macrophages, which produce elevated levels of cytokines (TNF-α, IL-6, IL-1β) and an amplification of disease-causing the so-called “cytokine storm” [95, 96]. Compared with moderate CoVID-19, severe cases more frequently have higher levels of CRP, ferritin, IL-6, IL-10, and TNF-α [88]. Absolute numbers of T lymphocytes, CD4 + T cells, and CD8 + T cells decrease in all the patients, and mainly in severe cases. Moreover, severe CoVID-19 has been associated with lower expression of IFN-γ by CD4+, an antiviral protein [97]. It was demonstrated that patients with obesity and overweight presented higher levels of CRP and ferritin than patients with normal weight, in a CoVID-19 medical ward, whereas IL-6 levels were higher in all the subjects [98].
In conclusion, the cytokines storm promoted by SARS-CoV-2 could act synergistically with the low-grade inflammation present in obesity to develop a severe CoVID-19.
Lipid metabolism
Free fatty acids (FFAs) from diet and from adipose tissue represent a source of reactive oxygen species, leading to the synthesis of TNF-α, IL-1β, IL-6, and the activation of cyclooxygenase-2 (COX-2), the latter increasing the production of prostaglandins [99, 100]. On the other hand, TNF-α and other cytokines stimulated lipolysis and release of FFAs and, thus, sustaining inflammation [101]. We can assume that FFAs can directly support CoVID-19 in individual with obesity. In fact, viral infections mobilize FFAs to support capsid-associated membrane formation. It was demonstrated that in hospitalized patients with severe pneumonia, CoVID-19 is independently associated with higher FFAs [102]. Indeed, CoVID-19 infection increases circulating levels of FFAs, which are correlated with clinical laboratory markers of inflammation (IL-6 and CRP) [103]. Moreover, saturated fatty acids (mainly palmitic, lauric and stearic acids) induce inflammatory response through the Toll-like receptors (TLRs-4) signaling pathway with an overexpressed of TLRs both in T2DM and in obesity [100]. The driver is inflammasome, that favors the pro-inflammatory IL-1β production [100]. Indeed, it was suggested that the spike protein interacts with human TLRs, especially TLR-4, suggesting an increased susceptibility to viral infection in patients with higher levels of fatty acids [104]. Furthermore, HDL-scavenger receptor B type 1 (SR-B1) has been shown to promote SARS-CoV-2 entry into cells expressing ACE2 [105]. It could be suggested that a higher consumption of HDL and/or the upregulation of SR-B1 expression is associated with CoVID-19 origin.
Meta-inflammation and fat embolism syndrome
Fat embolism syndrome was suggested as another mechanism expanding the major risk of severe disease in patients with obesity [106]. SARS-CoV-2-infected adipocytes could undergo necrotic death, as shown in other tissues, producing lipid remnant accumulation that could trigger fat embolism in the microcirculation. Fat embolism syndrome is a meta-inflammatory condition, where the increased expression of inflammatory mediators in adipose tissue, mainly due to macrophage infiltration, interferes with glucose metabolism. Lung autopsy of two overweight patients with CoVID-19 showed fat embolism macrophages infiltration in the adipose tissue [106].
Hypercoagulopathy
It is known that the expression of plasminogen activator inhibitor 1 (PAI-1), an inhibitor of fibrinolysis, is upregulated in patients with obesity [94]. Indeed, patients with overweight and obesity present an increased risk for thromboembolism [107]. It has been reported that various hemostatic system components like platelets, thrombin, and coagulation factors act as chemotactic for the immune system cells, resulting in an increased of various pro-inflammatory cytokines [108]. IL-1α, which is expressed by platelets, endothelial cells, and monocytes during pro-inflammatory conditions, represents a link between coagulation system and inflammatory response [108]. Moreover, it was demonstrated that patients with high IL-1β, IL-6, TNF-α, MCP-1 levels presented hypercoagulation and disseminated intravascular coagulation [109].
SARS-CoV-2 cytokine storm is strikingly linked to a state of hypercoagulability [110]. SARS-CoV-2 induces thrombin production and high levels of PAI-1, activating prothrombotic signaling pathways in platelets [79]. An endothelialitis, characterized by the presence of neutrophils and macrophages, was found in several vascular beds in patients with CoVID-19 and can support microthrombi deposition and cell damage [79]. With the expandability of adipose tissue, hypoxia may occur when there is not adequate neo-angiogenetic support, with the expression of Hypoxia-Induced Factor α (HIF-α), and, consequentially, adipocyte stress and death [90]. HIF-α signaling can be upregulated during an acute lung injury and this hyperproduction may contribute to the prothrombotic state [79].
Dysregulation of the renin–angiotensin–aldosterone system
In renin–angiotensin–aldosterone system (RAAS), renin converts angiotensinogen to angiotensin I, which is transformed to angiotensin II via ACE1, stimulating adrenal aldosterone secretion, renal retention of sodium and water and vasoconstriction [111]. Obesity is usually associated with dysregulated RAAS axis [112]. In fact, adipose tissue secretes angiotensinogen, mineralocorticoids, mineralocorticoid releasing factors such as leptin and cathepsins, which promote the enzymatic conversion of angiotensin I to angiotensin II. The IR and the endothelial dysfunction in patients with a dysfunctional adipose tissue lead to enhanced RAAS activation. Furthermore, obesity is associated with sympathetic nervous system activation, also by leptin, which stimulates renin secretion [112]. On the other hand, RAAS is implicated in acute lung injury and angiotensin II induces lung fibrosis [113]. SARS-CoV2 infects host cells via ACE2, causing the downstream of this receptor and angiotensin downregulation. These processes enhanced angiotensin II signaling and the adverse outcome in CoVID-19. Considering that lung injury is a hallmark of CoVID-19, it is possible that RAAS represents a link between obesity and CoVID-19 severity [113].
Beyond the above molecular mechanisms also pulmonary dysfunction, such as reduced lung volumes and compliance, and an increase in airway resistance may be trigger factors for the risk for CoVID-19 in patients with obesity [41, 114]. Indeed obesity-related comorbidities, such as CVD can worsen the outcome of CoVID-19 [41, 114].
Childhood
In the literature there are several hypotheses suggested to explain children’s lower risk of being affected by SARS-CoV-2 [115]. On the other hand, according to the currently available studies, few studies investigated the predisposing factors the worst prognosis in children with obesity and CoVID-19. Cellular tropism seen in adults seems to be of much less interest to children. In fact, it was demonstrated an age-dependent ACE2 gene expression in the nasal epithelium [116] and in pneumocytes [117]. It was recently showed that higher expression of CD147-related genes are correlated to a higher BMI and older age on immune cells, but not on the barrier cells which can determine the course of CoVID-19 [89].
Only few studies investigated the immune response to SARS-CoV-2 in children and very few in children with obesity. It has been shown that severe disease is associated with obesity in older children, with a pro-inflammatory profile characterized by a high CRP and IL-6 levels at admission [118]. The multisystem inflammatory syndrome in children associated with CoVID-19 (MIS-C) and determined by the presence of SARS-CoV-2 antibodies, may represent an immune-mediated complication of SARS-CoV-2 infection in children and adolescents [119]. Interestingly, in a study in which has been shown a high prevalence of a genetic variant impairing negative regulation of interferon and inflammatory signaling 8 out of 18 patients with MIS-C (44%) were affected by obesity [120].
Conclusions
The phenomenon of the CoVID-19 inflammation can be amplified in subjects of obesity according to different mechanisms that seem to interact to each other to induce the worst prognosis. Most children appear to be protected from an acute hyperinflammatory response to SARS-CoV-2. Among the predisposing factors that explain the susceptibility and severity of the clinical disease CoVID-19, obesity represents the principal co-morbidity impairing an efficient immune response. In conclusion, further studies are mandatory to confirm the role of low-grade chronic inflammation in clinical aspects of CoVID-19 in subjects with obesity.
Early identification of low-grade inflammation could be fundamental and should be decision-making regarding hospitalization, early respiratory support, and therapy with immunosuppression to improve the severity of CoVID-19.
References
Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–60.
Collaboration NCDRF. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–96.
WHO. Obesity and overweight. 2016; https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
Michalakis K, Goulis DG, Vazaiou A, Mintziori G, Polymeris A, Abrahamian-Michalakis A. Obesity in the ageing man. Metabolism. 2013;62:1341–9.
Barrea L, Pugliese G, Framondi L, Di Matteo R, Laudisio D, Savastano S, et al. Does Sars-Cov-2 threaten our dreams? Effect of quarantine on sleep quality and body mass index. J Transl Med. 2020;18:318.
Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6:944–53.
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918.
Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8:616–27.
Stefan N, Haring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7:313–24.
Donohoe CL, Lysaght J, O’Sullivan J, Reynolds JV. Emerging concepts linking obesity with the hallmarks of cancer. Trends Endocrinol Metab. 2017;28:46–62.
Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–85.
Kloting N, Bluher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014;15:277–87.
Louwen F, Ritter A, Kreis NN, Yuan J. Insight into the development of obesity: functional alterations of adipose-derived mesenchymal stem cells. Obes Rev. 2018;19:888–904.
Febbraio MA. Role of interleukins in obesity: implications for metabolic disease. Trends Endocrinol Metab. 2014;25:312–9.
Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–43.
Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6.
McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest. 2017;127:5–13.
Exley MA, Hand L, O’Shea D, Lynch L. Interplay between the immune system and adipose tissue in obesity. J Endocrinol. 2014;223:R41–8.
Ghilotti F, Bellocco R, Ye W, Adami HO, Trolle Lagerros Y. Obesity and risk of infections: results from men and women in the Swedish National March Cohort. Int J Epidemiol. 2019;48:1783–94.
Honce R, Schultz-Cherry S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol. 2019;10:1071.
Huttunen R, Syrjanen J. Obesity and the risk and outcome of infection. Int J Obes. 2013;37:333–40.
Maier HE, Lopez R, Sanchez N, Ng S, Gresh L, Ojeda S, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis. 2018;218:1378–82.
Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985.
Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–6.
Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966.
Kim J, Nam JH. Insight into the relationship between obesity-induced low-level chronic inflammation and COVID-19 infection. Int J Obes. 2020;44:1541–2.
Frasca D, Reidy L, Cray C, Diaz A, Romero M, Kahl K, et al. Influence of obesity on serum levels of SARS-CoV-2-specific antibodies in COVID-19 patients. PLoS ONE. 2021;16:e0245424.
Frasca D, Reidy L, Romero M, Diaz A, Cray C, Kahl K, et al. The majority of SARS-CoV-2-specific antibodies in COVID-19 patients with obesity are autoimmune and not neutralizing. Int J Obes. 2022;46:427–32.
Gotzinger F, Santiago-Garcia B, Noguera-Julian A, Lanaspa M, Lancella L, Calo Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–61.
Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–64.
CDC. Hospitalizations Associated with COVID-19 Among Children and Adolescents — COVID-NET, 14 States, March 1, 2020–August 14, 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7036e2.htm. Accessed February 3, 2022.
Dong Y, Mo X, Hu Y, Qi X, Jiang F, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702.
Panahi L, Amiri M, Pouy S. Clinical characteristics of COVID-19 infection in newborns and pediatrics: a systematic review. Arch Acad Emerg Med. 2020;8:e50.
Fruhbeck G, Baker JL, Busetto L, Dicker D, Goossens GH, Halford JCG, et al. European association for the study of obesity position statement on the global COVID-19 pandemic. Obes Facts. 2020;13:292–6.
Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017;92:251–65.
Alwarawrah Y, Kiernan K, MacIver NJ. Changes in nutritional status impact immune cell metabolism and function. Front Immunol. 2018;9:1055.
Wu CP, Adhi F, Highland K. Recognition and management of respiratory co-infection and secondary bacterial pneumonia in patients with COVID-19. Cleve Clin J Med. 2020;87:659–63.
Al Heialy S, Hachim MY, Senok A, Gaudet M, Abou Tayoun A, Hamoudi R, et al. Regulation of angiotensin-converting enzyme 2 in obesity: implications for COVID-19. Front Physiol. 2020;11:555039.
Zhang F, Xiong Y, Wei Y, Hu Y, Wang F, Li G, et al. Obesity predisposes to the risk of higher mortality in young COVID-19 patients. J Med Virol. 2020;92:2536–42.
Kass DA, Duggal P, Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395:1544–5.
Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21:e13128.
Collaboration NCDRF. Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569:260–4.
Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet. 2020;395:65–74.
Albashir AAD. The potential impacts of obesity on COVID-19. Clin Med. 2020;20:e109–e13.
Muscogiuri G, Pugliese G, Barrea L, Savastano S, Colao A. Commentary: obesity: the “Achilles heel” for COVID-19? Metabolism. 2020;108:154251.
Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;1–8.
Eastment MC, Berry K, Locke E, Green P, O’Hare A, Crothers K, et al. BMI and outcomes of SARS-CoV-2 among US veterans. Obesity. 2021;29:900–8.
Kim SY, Yoo DM, Min C, Wee JH, Kim JH, Choi HG. Analysis of mortality and morbidity in COVID-19 patients with obesity using clinical epidemiological data from the korean center for disease control & prevention. Int J Environ Res Public Health. 2020;17:24.
Wang J, Zhu L, Liu L, Zhao XA, Zhang Z, Xue L, et al. Overweight and obesity are risk factors of severe illness in patients with COVID-19. Obesity. 2020;28:2049–55.
Breland JY, Wong MS, Steers WN, Yuan AH, Haderlein TP, Washington DL. BMI and risk for severe COVID-19 among veterans health administration patients. Obesity. 2021;29:825–8.
Recalde M, Pistillo A, Fernandez-Bertolin S, Roel E, Aragon M, Freisling H, et al. Body mass index and risk of COVID-19 diagnosis, hospitalisation, and death: a cohort study of 2 524 926 Catalans. J Clin Endocrinol Metab. 2021;106:e5030–42.
Sales-Peres SHC, de Azevedo-Silva LJ, Bonato RCS, Sales-Peres MC, Pinto A, Santiago Jr JF. Coronavirus (SARS-CoV-2) and the risk of obesity for critically illness and ICU admitted: meta-analysis of the epidemiological evidence. Obes Res Clin Pract. 2020;14:389–97.
Onder G, Palmieri L, Vanacore N, Giuliano M, Brusaferro S. Italian National Institute of Health C-MG. Nonrespiratory complications and obesity in patients dying with COVID-19 in Italy. Obesity. 2021;29:20–3.
Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16:341–2.
Gao F, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care. 2020;43:e72–e4.
Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195–9.
Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71:896–7.
Mahrooz A, Muscogiuri G, Buzzetti R, Maddaloni E. The complex combination of COVID-19 and diabetes: pleiotropic changes in glucose metabolism. Endocrine. 2021;72:317–25.
Epsi NJ, Richard SA, Laing ED, Fries AC, Millar E, Simons MP, et al. Clinical, immunological and virological SARS-CoV-2 phenotypes in obese and non-obese military health system beneficiaries. J Infect Dis. 2021;224:1462–72.
Yang Y, Ding L, Zou X, Shen Y, Hu D, Hu X, et al. Visceral adiposity and high intramuscular fat deposition independently predict critical illness in patients with SARS-CoV-2. Obesity. 2020;28:2040–8.
Andrade FB, Gualberto A, Rezende C, Percegoni N, Gameiro J, Hottz ED. The weight of obesity in immunity from influenza to COVID-19. Front Cell Infect Microbiol. 2021;11:638852.
Busetto L, Bettini S, Fabris R, Serra R, Dal Pra C, Maffei P, et al. Obesity and COVID-19: an Italian snapshot. Obesity. 2020;28:1600–5.
Malik VS, Ravindra K, Attri SV, Bhadada SK, Singh M. Higher body mass index is an important risk factor in COVID-19 patients: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2020;27:42115–23.
Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262.
Fresan U, Guevara M, Elia F, Albeniz E, Burgui C, Castilla J, et al. Independent role of severe obesity as a risk factor for COVID-19 hospitalization: a Spanish Population-Based Cohort Study. Obesity. 2021;29:29–37.
Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O’Rahilly S, Aveyard P, et al. Associations between body-mass index and COVID-19 severity in 6.9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9:350–9.
Battisti S, Pedone C, Napoli N, Russo E, Agnoletti V, Nigra SG, et al. Computed tomography highlights increased visceral adiposity associated with critical illness in COVID-19. Diabetes Care. 2020;43:e129–e30.
Watanabe M, Caruso D, Tuccinardi D, Risi R, Zerunian M, Polici M, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111:154319.
Petersen A, Bressem K, Albrecht J, Thiess HM, Vahldiek J, Hamm B, et al. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317.
Kara AA, Boncuoglu E, Kiymet E, Arikan KO, Sahinkaya S, Duzgol M, et al. Evaluation of predictors of severe-moderate COVID-19 infections at children: a review of 292 children. J Med Virol. 2021;93:6634–40.
Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–77.
Karakaya Molla G, Unal Uzun O, Koc N, Ozen Yesil B, Bayhan GI. Evaluation of nutritional status in pediatric patients diagnosed with Covid-19 infection. Clin Nutr ESPEN. 2021;44:424–8.
Sandoval M, Nguyen DT, Vahidy FS, Graviss EA. Risk factors for severity of COVID-19 in hospital patients age 18-29 years. PLoS ONE. 2021;16:e0255544.
Badedi M, Darraj H, Alnami AQ, Makrami A, Mahfouz MS, Alhazmi K, et al. Epidemiological and clinical characteristics of deceased COVID-19 patients. Int J Gen Med. 2021;14:3809–19.
Caballero B. Humans against obesity: who will win? Adv Nutr. 2019;10(suppl_1):S4–S9.
Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13:707–12.
Morgan OW, Bramley A, Fowlkes A, Freedman DS, Taylor TH, Gargiullo P, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS ONE. 2010;5:e9694.
Paich HA, Sheridan PA, Handy J, Karlsson EA, Schultz-Cherry S, Hudgens MG, et al. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity. 2013;21:2377–86.
Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–32.
Kruglikov IL, Scherer PE. The role of adipocytes and adipocyte-like cells in the severity of COVID-19 infections. Obesity. 2020;28:1187–90.
Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, et al. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295:R781–8.
Maurya R, Sebastian P, Namdeo M, Devender M, Gertler A. COVID-19 severity in obesity: leptin and inflammatory cytokine interplay in the link between high morbidity and mortality. Front Immunol. 2021;12:649359.
Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–9.
Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes Med. 2020;19:100283.
Bellido V, Perez A. Inpatient hyperglycemia management and COVID-19. Diabetes Ther. 2021;12:121–32.
Martyn JA, Kaneki M, Yasuhara S. Obesity-induced insulin resistance and hyperglycemia: etiologic factors and molecular mechanisms. Anesthesiology. 2008;109:137–48.
Sarver DC, Wong GW. Obesity alters Ace2 and Tmprss2 expression in lung, trachea, and esophagus in a sex-dependent manner:Implications for COVID-19. Biochem Biophy Res Commun. 2021;538:92–6.
Wang K, Chen W, Zhang Z, Deng Y, Lian JQ, Du P, et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5:283.
Radzikowska U, Ding M, Tan G, Zhakparov D, Peng Y, Wawrzyniak P, et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020;75:2829–45.
Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–101.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84.
Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55.
Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014;1842:446–62.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97.
Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020;80:607–13.
Muscogiuri G, Pugliese G, Laudisio D, Castellucci B, Barrea L, Savastano S, et al. The impact of obesity on immune response to infection: Plausible mechanisms and outcomes. Obes Rev. 2021;22:e13216.
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–9.
Bettini S, Bucca G, Sensi C, Dal Pra C, Fabris R, Vettor R, et al. Higher levels of C-reactive protein and ferritin in patients with overweight and obesity and SARS-CoV-2-related pneumonia. Obes Facts. 2021;14:543–9.
Ghosh A, Gao L, Thakur A, Siu PM, Lai CWK. Role of free fatty acids in endothelial dysfunction. J Biomed Sci. 2017;24:50.
Rogero MM, Calder PC. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients. 2018;10:432.
Zelova H, Hosek J. TNF-alpha signalling and inflammation: interactions between old acquaintances. Inflamm Res. 2013;62:641–51.
Nguyen M, Bourredjem A, Piroth L, Bouhemad B, Jalil A, Pallot G, et al. High plasma concentration of non-esterified polyunsaturated fatty acids is a specific feature of severe COVID-19 pneumonia. Sci Rep. 2021;11:10824.
Thomas T, Stefanoni D, Reisz JA, Nemkov T, Bertolone L, Francis RO, et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020;5:e140327.
Brandao SCS, Ramos JOX, Dompieri LT, Godoi E, Figueiredo JL, Sarinho ESC, et al. Is Toll-like receptor 4 involved in the severity of COVID-19 pathology in patients with cardiometabolic comorbidities? Cytokine Growth Factor Rev. 2021;58:102–10.
Wei C, Wan L, Yan Q, Wang X, Zhang J, Yang X, et al. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat Metab. 2020;2:1391–400.
Cinti S, Graciotti L, Giordano A, Valerio A, Nisoli E. COVID-19 and fat embolism: a hypothesis to explain the severe clinical outcome in people with obesity. Int J Obes. 2020;44:1800–2.
Hotoleanu C. Association between obesity and venous thromboembolism. Med Pharm Rep. 2020;93:162–8.
Cavalli G, Colafrancesco S, Emmi G, Imazio M, Lopalco G, Maggio MC, et al. Interleukin 1alpha: a comprehensive review on the role of IL-1alpha in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun Rev. 2021;20:102763.
Walborn A, Hoppensteadt D, Syed D, Mosier M, Fareed J. Biomarker profile of sepsis-associated coagulopathy using biochip assay for inflammatory cytokines. Clin Appl Thromb Hemost. 2018;24:625–32.
Savla SR, Prabhavalkar KS, Bhatt LK. Cytokine storm associated coagulation complications in COVID-19 patients: pathogenesis and management. Expert Rev Anti Infect Ther. 2021;19:1397–413.
Ames MK, Atkins CE, Pitt B. The renin-angiotensin-aldosterone system and its suppression. J Vet Intern Med. 2019;33:363–82.
Cabandugama PK, Gardner MJ, Sowers JR. The renin angiotensin aldosterone system in obesity and hypertension: roles in the cardiorenal metabolic syndrome. Med Clin North Am. 2017;101:129–37.
Akoumianakis I, Filippatos T. The renin-angiotensin-aldosterone system as a link between obesity and coronavirus disease 2019 severity. Obes Rev. 2020;21:e13077.
Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected-obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol. 2021;17:135–49.
Williams PCM, Howard-Jones AR, Hsu P, Palasanthiran P, Gray PE, McMullan BJ, et al. SARS-CoV-2 in children: spectrum of disease, transmission and immunopathological underpinnings. Pathology. 2020;52:801–8.
Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. J Am Med Assoc. 2020;323:2427–9.
Lee PI, Hu YL, Chen PY, Huang YC, Hsueh PR. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53:371–2.
Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children’s hospital in New York City, New York. JAMA Pediatr. 2020;174:e202430.
Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–8.
Chou J, Platt CD, Habiballah S, Nguyen AA, Elkins M, Weeks S, et al. Mechanisms underlying genetic susceptibility to multisystem inflammatory syndrome in children (MIS-C). J Allergy Clin Immunol. 2021;148:732–8 e1.
Acknowledgements
The scientific assistance of Panta Rei Impresa Sociale srl (https://www.panta-rei.eu/pantarei/) is gratefully appreciated. We acknowledge Angelo Ferrara for collecting data.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization: GM, SS, and AC; methodology: GM; validation: GM; formal analysis: GM; investigation and data curation: SB, MB, and LB; writing—original draft preparation: LB, GM, SB, and MB; writing—review and editing: LB and GM; visualization: AC and SS; supervision: AC and SS. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muscogiuri, G., Bettini, S., Boschetti, M. et al. Low-grade inflammation, CoVID-19, and obesity: clinical aspect and molecular insights in childhood and adulthood. Int J Obes 46, 1254–1261 (2022). https://doi.org/10.1038/s41366-022-01111-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-022-01111-5
This article is cited by
-
Human coronavirus OC43-elicited CD4+ T cells protect against SARS-CoV-2 in HLA transgenic mice
Nature Communications (2024)