Abstract
Background
Genetically modified probiotics have potential for use as a novel approach to express bioactive molecules for the treatment of obesity. The objective of the present study was to investigate the beneficial effect of genetically modified Escherichia coli Nissle 1917 (EcN-GM) in obese C57BL/6J mice.
Methods
First, an obesity model in C57BL/6J mice was successfully established. Then, the obese mice were randomly assigned into three groups: obese mice (OB), obese mice + EcN-GM (OB + EcN-GM), and obese mice + orlistat (OB + orlistat) (n = 10 in each group). The three groups were gavaged with 0.3 ml of 1010 CFU/ml control EcN, EcN-GM (genetically engineered EcN) and 10 ml/kg orlistat. Body weight, food consumption, fat pad and organ weight, hepatic biochemistry and hepatic histopathological alterations were measured. The effects of EcN-GM on the levels of endocrine peptides and the intestinal microbiota were also analyzed.
Results
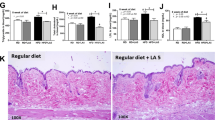
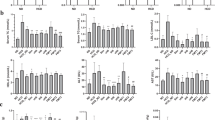
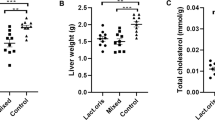
After supplementation for 8 weeks, EcN-GM was associated with decreases in body weight gain, food intake, fat pad and liver weight, and alleviation hepatocyte steatosis in obese mice. EcN-GM also increased the level of GLP-1 in serum and alleviated leptin and insulin resistance. Moreover, supplementation with EcN-GM increased the α-diversity of the intestinal microbiota but did not significantly influence the relative abundance of Firmicutes and Bacteroidetes.
Conclusions
These results indicated that EcN-GM, a genetically modified E. coli strain, may be a potential therapeutic approach to treat obesity. The beneficial effect of EcN-GM may be independent of the alteration of the diversity and composition of the intestinal microbiota in obese mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kyle TK, Dhurandhar EJ, Allison DB. Regarding obesity as a disease: evolving policies and their implications. Endocrinol Metab Clin North Am. 2016;45:511–20.
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253.
Bray GA, Kim KK, Wilding JPH, World Obesity F. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18:715–23.
O’Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392:637–49.
Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342–62.
Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018;14:12–24.
Bessesen DH, Van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018;6:237–48.
Komaroff AL. The microbiome and risk for obesity and diabetes. JAMA. 2017;317:355–6.
Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254–66.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31.
Pedrolli DB, Ribeiro NV, Squizato PN, de Jesus VN, Cozetto DA, Team AQAUai. Engineering microbial living therapeutics: the synthetic biology toolbox. Trends Biotechnol. 2019;37:100–15.
Reardon S. Genetically modified bacteria enlisted in fight against disease. Nature. 2018;558:497–8.
Hendrikx T, Duan Y, Wang Y, Oh JH, Alexander LM, Huang W, et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut. 2019;68:1504–15.
Chen Z, Guo L, Zhang Y, Walzem RL, Pendergast JS, Printz RL, et al. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J Clin Invest. 2014;124:3391–406.
Wassenaar TM. Insights from 100 years of research with probiotic E. Coli. Eur J Microbiol Immunol. 2016;6:147–61.
Grozdanov L, Raasch C, Schulze J, Sonnenborn U, Gottschalk G, Hacker J, et al. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J Bacteriol. 2004;186:5432–41.
Hancock V, Dahl M, Klemm P. Probiotic Escherichia coli strain Nissle 1917 outcompetes intestinal pathogens during biofilm formation. J Med Microbiol. 2010;59:392–9.
Kurtz CB, Millet YA, Puurunen MK, Perreault M, Charbonneau MR, Isabella VM. et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci Transl Med. 2019;11:eaau7975.
Isabella VM, Ha BN, Castillo MJ, Lubkowicz DJ, Rowe SE, Millet YA, et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat Biotechnol. 2018;36:857–64.
Hwang IY, Koh E, Wong A, March JC, Bentley WE, Lee YS, et al. Engineered probiotic Escherichia coli can eliminate and prevent Pseudomonas aeruginosa gut infection in animal models. Nat Commun. 2017;8:15028.
Ma J, Li C, Wang J, Gu J. Genetically engineered Escherichia coli Nissle 1917 secreting GLP-1 analog exhibits potential antiobesity effect in high-fat diet-induced obesity mice. Obesity. 2020;28:315–22.
Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8:402–24.
Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27.
Reid G, Sanders ME, Gaskins HR, Gibson GR, Mercenier A, Rastall R, et al. New scientific paradigms for probiotics and prebiotics. J Clin Gastroenterol. 2003;37:105–18.
Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288:25088–97.
Kang JH, Yun SI, Park MH, Park JH, Jeong SY, Park HO. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS ONE. 2013;8:e54617.
Valsecchi C, Carlotta Tagliacarne S, Castellazzi A. Gut microbiota and obesity. J Clin Gastroenterol. 2016;50:S157–8. Suppl 2Proceedings from the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health meeting held in Rome, Italy on September 13–15, 2015.
Wei P, Yang Y, Li T, Ding Q, Sun H. A engineered Bifidobacterium longum secreting a bioative penetratin-Glucagon-like peptide 1 fusion protein enhances Glucagon-like peptide 1 absorption in the intestine. J Microbiol Biotechnol. 2015. Epub ahead of print.
Duan FF, Liu JH, March JC. Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes. Diabetes. 2015;64:1794–803.
Rao S, Hu S, McHugh L, Lueders K, Henry K, Zhao Q, et al. Toward a live microbial microbicide for HIV: commensal bacteria secreting an HIV fusion inhibitor peptide. Proc Natl Acad Sci USA. 2005;102:11993–8.
Federico A, Dallio M, Rosa DIS, Giorgio V, Miele L. Gut microbiota, obesity and metabolic disorders. Minerva Gastroenterol Dietol. 2017;63:337–44.
Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–23.
Boulange CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8:42.
Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross RP, Fitzgerald GF, et al. Gut microbiota, obesity and diabetes. Postgrad Med J. 2016;92:286–300.
Madsen MSA, Holm JB, Palleja A, Wismann P, Fabricius K, Rigbolt K, et al. Metabolic and gut microbiome changes following GLP-1 or dual GLP-1/GLP-2 receptor agonist treatment in diet-induced obese mice. Sci Rep. 2019;9:15582.
Zhang N, Tao J, Gao L, Bi Y, Li P, Wang H, et al. Liraglutide attenuates nonalcoholic fatty liver disease by modulating gut microbiota in rats administered a high-fat diet. Biomed Res Int. 2020;2020:2947549.
Liu Q, Cai BY, Zhu LX, Xin X, Wang X, An ZM, et al. Liraglutide modulates gut microbiome and attenuates nonalcoholic fatty liver in db/db mice. Life Sci. 2020;261:118457.
Ehrlich SD. Probiotics—little evidence for a link to obesity. Nat Rev Microbiol. 2009;7:901.
Daniel H, Gholami AM, Berry D, Desmarchelier C, Hahne H, Loh G, et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014;8:295–308.
Kanoski SE, Hayes MR, Skibicka KP. GLP-1 and weight loss: unraveling the diverse neural circuitry. Am J Physiol Regul Integr Comp Physiol. 2016;310:R885–95.
Author information
Authors and Affiliations
Contributions
All authors contributed to this work. JM, JW, and JG designed the experiment. JM and JW performed the experiment. LX and YL analyzed the data. JM and JW drafted the manuscript. JM, LX, and YL prepared the figures. JM, JW, LX, and JG critically revised the manuscript. All the listed authors reviewed and approved the submitted manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, J., Wang, J., Xu, L. et al. The beneficial effects of genetically engineered Escherichia coli Nissle 1917 in obese C57BL/6J mice. Int J Obes 46, 1002–1008 (2022). https://doi.org/10.1038/s41366-022-01073-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-022-01073-8