Abstract
Background
Milk-fat globule membrane (MFGM) is a complex structure secreted by the mammary gland and present in mammalian milk. MFGM contains lipids and glycoproteins as well as gangliosides, which may be involved in myelination processes. Notably, myelination and thereby white matter integrity are often altered in obesity. Furthermore, MFGM interventions showed beneficial effects in obesity by affecting inflammatory processes and the microbiome. In this study, we investigated the impact of a dietary MFGM intervention on fat storage, neuroinflammatory processes and myelination in a rodent model of high fat diet (HFD)-induced obesity.
Methods
12-week-old male low density lipoprotein receptor-deficient Leiden mice were exposed to a HFD, a HFD enriched with 3% whey protein lipid concentrate (WPC) high in MFGM components, or a low fat diet. The impact of MFGM supplementation during 24-weeks of HFD-feeding was examined over time by analyzing body weight and fat storage, assessing cognitive tasks and MRI scanning, analyzing myelinization with polarized light imaging and examining neuroinflammation using immunohistochemistry.
Results
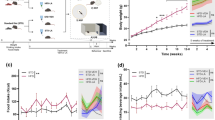
We found in this study that 24 weeks of HFD-feeding induced excessive fat storage, increased systolic blood pressure, altered white matter integrity, decreased functional connectivity, induced neuroinflammation and impaired spatial memory. Notably, supplementation with 3% WPC high in MFGM components restored HFD-induced neuroinflammation and attenuated the reduction in hippocampal-dependent spatial memory and hippocampal functional connectivity.
Conclusions
We showed that supplementation with WPC high in MFGM components beneficially contributed to hippocampal-dependent spatial memory, functional connectivity in the hippocampus and anti-inflammatory processes in HFD-induced obesity in rodents. Current knowledge regarding exact biological mechanisms underlying these effects should be addressed in future studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Manno FAM, Isla AG, Manno SHC, Ahmed I, Cheng SH, Barrios FA, et al. Early stage alterations in white matter and decreased functional interhemispheric hippocampal connectivity in the 3xTg mouse model of alzheimer’s disease. Front Aging Neurosci. 2019;11:39.
Moukarzel S, Dyer RA, Garcia C, Wiedeman AM, Boyce G, Weinberg J, et al. Milk fat globule membrane supplementation in formula-fed rat pups improves reflex development and may alter brain lipid composition. Scientific Rep. 2018;8:15277.
Timby N, Domellof E, Hernell O, Lonnerdal B, Domellof M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial. Am J Clin Nutr. 2014;99:860–8.
Zhang D, Wen J, Zhou J, Cai W, Qian L. Milk fat globule membrane ameliorates necrotizing enterocolitis in neonatal rats and suppresses lipopolysaccharide-induced inflammatory response in IEC-6 enterocytes. JPEN J Parenter Enteral Nutr. 2019;43:863–73.
Oshida K, Shimizu T, Takase M, Tamura Y, Shimizu T, Yamashiro Y. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatr Res. 2003;53:589–93.
Vickers MH, Guan J, Gustavsson M, Krageloh CU, Breier BH, Davison M, et al. Supplementation with a mixture of complex lipids derived from milk to growing rats results in improvements in parameters related to growth and cognition. Nutr Res. 2009;29:426–35.
Milard M, Laugerette F, Durand A, Buisson C, Meugnier E, Loizon E, et al. Milk polar lipids in a high-fat diet can prevent body weight gain: modulated abundance of gut bacteria in relation with fecal loss of specific fatty acids. Mol Nutr Food Res. 2019;63:e1801078.
Demmer E, Van Loan MD, Rivera N, Rogers TS, Gertz ER, German JB, et al. Addition of a dairy fraction rich in milk fat globule membrane to a high-saturated fat meal reduces the postprandial insulinaemic and inflammatory response in overweight and obese adults. J Nutr Sci. 2016;5:e14.
Brink LR, Gueniot JP, Lönnerdal B. Effects of milk fat globule membrane and its various components on neurologic development in a postnatal growth restriction rat model. J Nutr Biochem. 2019;69:163–71.
Lee H, Padhi E, Hasegawa Y, Larke J, Parenti M, Wang A, et al. Compositional dynamics of the milk fat globule and its role in infant development. Front Pediatrics. 2018;6:313.
McJarrow P, Schnell N, Jumpsen J, Clandinin T. Influence of dietary gangliosides on neonatal brain development. Nutr Rev. 2009;67:451–63.
Palmano K, Rowan A, Guillermo R, Guan J, McJarrow P. The role of gangliosides in neurodevelopment. Nutrients. 2015;7:3891–913.
Arnoldussen IA, Kiliaan AJ, Gustafson DR. Obesity and dementia: adipokines interact with the brain. Eur Neuropsychopharmacol. 2014;24:1982–99.
Arnoldussen IAC, Wiesmann M, Pelgrim CE, Wielemaker EM, van Duyvenvoorde W, Amaral-Santos PL, et al. Butyrate restores HFD-induced adaptations in brain function and metabolism in mid-adult obese mice. Int J Obes (Lond). 2017;41:935–44.
Pelgrim CE, Franx BAA, Snabel J, Kleemann R, Arnoldussen IAC, Kiliaan AJ. Butyrate reduces HFD-induced adipocyte hypertrophy and metabolic risk factors in obese LDLr−/−. Leiden Mice. Nutrients. 2017;9:1–15.
Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ. Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol. 2008;63:652–7.
Pannacciulli N, Del Parigi A, Chen K, Le DSN, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–25.
Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–64.
Sui SX, Pasco JA. Obesity and brain function: the brain-body crosstalk. Medicina (Kaunas). 2020;56:499.
Van Opstal A, Wijngaarden M, van der Grond J, Pijl H. Changes in brain activity after weight loss. Obes Sci Pract. 2019;5:459–67.
Yau PL, Kang EH, Javier DC, Convit A. Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity. 2014;22:1865–71.
Morrison MC, Mulder P, Salic K, Verheij J, Liang W, van Duyvenvoorde W, et al. Intervention with a caspase-1 inhibitor reduces obesity-associated hyperinsulinemia, non-alcoholic steatohepatitis and hepatic fibrosis in LDLR−/−. Leiden mice. Int J Obes (Lond). 2016;40:1416–23.
Mueller AM, Kleemann R, Gart E, van Duyvenvoorde W, Verschuren L, Caspers M, et al. Cholesterol accumulation as a driver of hepatic inflammation under translational dietary conditions can be attenuated by a multicomponent medicine. Front Endocrinol. 2021;12:601160.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Plos Biol. 2010;8:1–5.
Brink LR, Herren AW, McMillen S, Fraser K, Agnew M, Roy N, et al. Omics analysis reveals variations among commercial sources of bovine milk fat globule membrane. J Dairy Sci. 2020;103:3002–16.
Luque-Sierra A, Alvarez-Amor L, Kleemann R, Martín F, Varela LM. Extra-virgin olive oil with natural phenolic content exerts an anti-inflammatory effect in adipose tissue and attenuates the severity of atherosclerotic lesions in Ldlr−/−. Leiden Mice. Mol Nutr Food Res. 2018;62:e1800295.
Schoemaker MH, Kleemann R, Morrison MC, Verheij J, Salic K, van Tol EAF, et al. A casein hydrolysate based formulation attenuates obesity and associated non-alcoholic fatty liver disease and atherosclerosis in LDLr−/−. Leiden mice. PLoS ONE. 2017;12:e0180648.
Tengeler AC, Gart E, Wiesmann M, Arnoldussen IAC, van Duyvenvoorde W, Hoogstad M, et al. Propionic acid and not caproic acid, attenuates nonalcoholic steatohepatitis and improves (cerebro) vascular functions in obese Ldlr(−/−). Leiden mice. FASEB J. 2020;34:9575–93.
Jakobsdottir G, Xu J, Molin G, Ahrne S, Nyman M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS ONE. 2013;8:e80476.
Liu C, Li P, Li H, Wang S, Ding L, Wang H, et al. TREM2 regulates obesity-induced insulin resistance via adipose tissue remodeling in mice of high-fat feeding. J Transl Med. 2019;17:300.
Pei Y, Li H, Cai Y, Zhou J, Luo X, Ma L, et al. Regulation of adipose tissue inflammation by adenosine 2A receptor in obese mice. J Endocrinol. 2018;239:365–76.
Gainey SJ, Kwakwa KA, Bray JK, Pillote MM, Tir VL, Towers AE, et al. Short-term high-fat diet (HFD) induced anxiety-like behaviors and cognitive impairment are improved with treatment by glyburide. Front Behav Neurosci. 2016;10:156.
Wang Q, Yuan J, Yu Z, Lin L, Jiang Y, Cao Z, et al. FGF21 attenuates high-fat diet-induced cognitive impairment via metabolic regulation and anti-inflammation of obese mice. Mol Neurobiol. 2018;55:4702–17.
Alghamdi BS. The effect of short-term feeding of a high-coconut oil or high-fat diet on neuroinflammation and the performance of an object–place task in rats. Neurochem Res. 2021;46:287–98.
Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trend Endocrinol Metab. 2013;24:40–7.
Cavaliere G, Trinchese G, Penna E, Cimmino F, Pirozzi C, Lama A, et al. High-fat diet induces neuroinflammation and mitochondrial impairment in mice cerebral cortex and synaptic fraction. Front Cell Neurosci. 2019;13:509–21.
Kullmann S, Callaghan MF, Heni M, Weiskopf N, Scheffler K, Häring HU, et al. Specific white matter tissue microstructure changes associated with obesity. Neuroimage. 2016;125:36–44.
Kullmann S, Schweizer F, Veit R, Fritsche A, Preissl H. Compromised white matter integrity in obesity. Obes Rev. 2015;16:273–81.
Samara A, Murphy T, Strain J, Rutlin J, Sun P, Neyman O, et al. Neuroinflammation and white matter alterations in obesity assessed by diffusion basis spectrum imaging. Front Hum Neurosci. 2019;13:464.
Huang HT, Tsai SF, Wu HT, Huang HY, Hsieh HH, Kuo YM, et al. Chronic exposure to high fat diet triggers myelin disruption and interleukin-33 upregulation in hypothalamus. BMC Neurosci. 2019;20:33.
Graham LC, Harder JM, Soto I, de Vries WN, John SWM, Howell GR. Chronic consumption of a western diet induces robust glial activation in aging mice and in a mouse model of Alzheimer’s disease. Scientific Rep. 2016;6:21568.
Graham LC, Grabowska WA, Chun Y, Risacher SL, Philip VM, Saykin AJ, et al. Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiol Aging.2019;80:154–72.
Graham LC, Kocalis HE, Soto I, Howell GR. Deficiency of complement component C1Q prevents cerebrovascular damage and white matter loss in a mouse model of chronic obesity. eNeuro.2020;86:154–72.
Zhou AL, Ward RE. Milk polar lipids modulate lipid metabolism, gut permeability, and systemic inflammation in high-fat-fed C57BL/6J ob/ob mice, a model of severe obesity. J Dairy Sci. 2019;102:4816–31.
Li T, Du M, Wang H, Mao X. Milk fat globule membrane and its component phosphatidylcholine induce adipose browning both in vivo and in vitro. J Nutr Biochem. 2020;81:108372.
Li T, Gao J, Du M, Song J, Mao X. Milk fat globule membrane attenuates high-fat diet-induced obesity by inhibiting adipogenesis and increasing uncoupling protein 1 expression in white adipose tissue of mice. Nutrients. 2018;10:331.
O’Mahony SM, McVey Neufeld KA, Waworuntu RV, Pusceddu MM, Manurung S, Murphy K, et al. The enduring effects of early-life stress on the microbiota-gut-brain axis are buffered by dietary supplementation with milk fat globule membrane and a prebiotic blend. Eur J Neurosci. 2020;51:1042–58.
Mudd AT, Alexander LS, Berding K, Waworuntu RV, Berg BM, Donovan SM, et al. Dietary prebiotics, milk fat globule membrane, and lactoferrin affects structural neurodevelopment in the young piglet. Front Pediatr.2016;4:1–10.
Fil JE, Fleming SA, Chichlowski M, Gross G, Berg BM, Dilger RN. Evaluation of dietary bovine milk fat globule membrane supplementation on growth, serum cholesterol and lipoproteins, and neurodevelopment in the young pig. Front Pediatr. 2019;7:417.
Gurnida DA, Rowan AM, Idjradinata P, Muchtadi D, Sekarwana N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum Dev. 2012;88:595–601.
Tanaka K, Hosozawa M, Kudo N, Yoshikawa N, Hisata K, Shoji H, et al. The pilot study: Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev. 2013;35:45–52.
Lopez PHH, Báez BB. Gangliosides in axon stability and regeneration. Prog Mol Biol Transl Sci. 2018;156:383–412.
McGonigal R, Barrie JA, Yao D, McLaughlin M, Cunningham ME, Rowan EG, et al. Glial sulfatides and neuronal complex gangliosides are functionally interdependent in maintaining myelinating axon integrity. J Neurosci. 2019;39:63–77.
Correa SG, Bianco ID, Riera CM, Fidelio GD. Anti-inflammatory effect of gangliosides in the rat hindpaw edema test. Eur J Pharmacol. 1991;199:93–8.
Acknowledgements
We thank Anouk Tengeler, Jos Dederen, Andor Veltien, Manuela van Rooij, Ferdinand Geus, Christian Smets, Bas de Cocq (all Radboudumc), Wim van Duyvenvoorde, and Jessica Snabel (all TNO Leiden) for their excellent scientific input. We thank the technicians of PRIME for their support in the execution of the experiment.
Funding
This research was funded by the Europees Fonds voor Regionale Ontwikkeling (EFRO), project BriteN 2016.
Author information
Authors and Affiliations
Contributions
Study design: RK, AK, GG, MM, JvD, and IA. Experimental work: IA, NPG, NW, MV, LvL, VV, and BG. Data analyses: IA, MW, and MM. Prepared full paper: IA, MM, MW, RK, and AJ. All authors reviewed the paper.
Corresponding author
Ethics declarations
Competing interests
Authors of Radboudumc and TNO have nothing to disclose. JAvD and GG are employees of Reckitt Mead Johnson Nutrition Institute.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Arnoldussen, I.A.C., Morrison, M.C., Wiesmann, M. et al. Milk fat globule membrane attenuates high fat diet-induced neuropathological changes in obese Ldlr−/−.Leiden mice. Int J Obes 46, 342–349 (2022). https://doi.org/10.1038/s41366-021-00998-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-00998-w
This article is cited by
-
High fat diet-induced obesity prolongs critical stages of the spermatogenic cycle in a Ldlr−/−.Leiden mouse model
Scientific Reports (2022)
-
Mouse models of nonalcoholic steatohepatitis and their application to new drug development
Archives of Pharmacal Research (2022)


