Abstract
Background
Early postnatal overfeeding (PO) induces long-term overweight and reduces brown adipose tissue (BAT) thermogenesis. Exercise has been suggested as a possible intervention to increase BAT function. In this study, we investigated chronical effects of moderate-intensity exercise in BAT function in postnatal overfed male Wistar rats
Methods
Litters’ delivery was on postnatal-day 0 - PN0. At PN2, litters were adjusted to nine (normal litter – NL) or three pups (small litter – SL) per dam. Animals were weaned on PN21 and in PN30 randomly divided into sedentary (NL-Sed and SL-Sed) or exercised (NL-Exe and SL-Exe), N of 14 litters per group. Exercise protocol started (PN30) with an effort test; training sessions were performed three times weekly at 60% of the VO2max achieved in effort test, until PN80. On PN81, a temperature transponder was implanted beneath the interscapular BAT, whose temperature was assessed in periods of lights-on and -off from PN87 to PN90. Sympathetic nerve activation of BAT was registered at PN90. Animals were euthanized at PN91 and tissues collected
Results
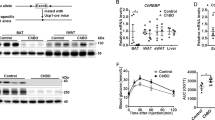
PO impaired BAT thermogenesis in lights-on (pPO < 0.0001) and -off (pPO < 0.01). Exercise increased BAT temperature in lights-on (pExe < 0.0001). In NL-Exe, increased BAT activity was associated with higher sympathetic activity (pExe < 0.05), β3-AR (pExe < 0.001), and UCP1 (pExe < 0.001) content. In SL-Exe, increasing BAT thermogenesis is driven by a combination of tissue morphology remodeling (pExe < 0.0001) with greater effect in increasing UCP1 (pExe < 0.001) and increased β3-AR (pExe < 0.001) content.
Conclusion
Moderate exercise chronically increased BAT thermogenesis in both, NL and SL groups. In NL-Exe by increasing Sympathetic activity, and in SL-Exe by a combination of increased β3-AR and UCP1 content with morphologic remodeling of BAT. Chronically increasing BAT thermogenesis in obese subjects may lead to higher overall energy expenditure, favoring the reduction of obesity and related comorbidities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nyberg ST, Batty GD, Pentti J, Virtanen M, Alfredsson L, Fransson EI, et al. Obesity and loss of disease-free years owing to major non-communicable diseases: a multicohort study. Lancet Public Health. 2018;3:e490–e497.
Plagemann A, Harder T, Schellong K, Schulz S, Stupin JH. Early postnatal life as a critical time window for determination of long-term metabolic health. Best Pract Res Clin Endocrinol Metab. 2012;26:641–53.
Jansen PW, Roza SJ, Jaddoe VW, Mackenbach JD, Raat H, Hofman A, et al. Children’s eating behavior, feeding practices of parents and weight problems in early childhood: results from the population-based Generation R Study. Int J Behav Nutr Phys Act. 2012;9:130.
Collaborators, LBDDBOM. Mapping local patterns of childhood overweight and wasting in low- and middle-income countries between 2000 and 2017. Nat Med. 2020;26:750–9.
Masters RK, Reither EN, Powers DA, Yang YC, Burger AE, Link BG. The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health. 2013;103:1895–901.
Habbout A, Li N, Rochette L, Vergely C. Postnatal overfeeding in rodents by litter size reduction induces major short- and long-term pathophysiological consequences. J Nutr. 2013;143:553–62.
de Almeida DL, Fabricio GS, Trombini AB, Pavanello A, Tofolo LP, da Silva Ribeiro TA, et al. Early overfeed-induced obesity leads to brown adipose tissue hypoactivity in rats. Cell Physiol Biochem. 2013;32:1621–30.
Chen KY, Brychta RJ, Abdul Sater Z, Cassimatis TM, Cero C, Fletcher LA, et al. Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity. J Biol Chem. 2020;295:1926–42.
Henry, BA and IJ Clarke, Chapter 14 - Animal Models for Manipulation of Thermogenesis, in Animal Models for the Study of Human Disease, PM Conn, Editor. 2013, Academic Press: Boston. p. 305–30.
Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, McGehee S, et al. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci U S A. 2017;114:8649–54.
Matsushita M, Yoneshiro T, Aita S, Kameya T, Sugie H, Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes (Lond). 2014;38:812–7.
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8.
Mirbolooki MR, Constantinescu CC, Pan ML, Mukherjee J. Quantitative assessment of brown adipose tissue metabolic activity and volume using 18F-FDG PET/CT and β3-adrenergic receptor activation. EJNMMI Res. 2011;1:30.
Vosselman MJ, van der Lans AA, Brans B, Wierts R, van Baak MA, Schrauwen P, et al. Systemic beta-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes. 2012;61:3106–13.
Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elia E, Kessler SH, Kahn PA, et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab. 2015;21:33–8.
Carey AL, Formosa MF, Van Every B, Bertovic D, Eikelis N, Lambert GW, et al. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia. 2013;56:147–55.
Lin YH, Chu LL. The health promotion lifestyle of metabolic syndrome individuals with a diet and exercise programme. Int J Nurs Pract. 2014;20:142–8.
Swift DL, McGee JE, Earnest CP, Carlisle E, Nygard M, Johannsen NM. The effects of exercise and physical activity on weight loss and maintenance. Prog Cardiovasc Dis. 2018;61:206–13.
De Matteis R, Lucertini F, Guescini M, Polidori E, Zeppa S, Stocchi V, et al. Exercise as a new physiological stimulus for brown adipose tissue activity. Nutr Metab Cardiovasc Dis. 2013;23:582–90.
Slocum N, Durrant JR, Bailey D, Yoon L, Jordan H, Barton J, et al. Responses of brown adipose tissue to diet-induced obesity, exercise, dietary restriction and ephedrine treatment. Exp Toxicol Pathol. 2013;65:549–57.
Moghetti P, Bacchi E, Brangani C, Dona S, Negri C. Metabolic effects of exercise. Front Horm Res. 2016;47:44–57.
Bassett DR, Craig BW. Influence of early nutrition on growth and adipose tissue characteristics in male and female rats. J Appl Physiol (1985). 1988;64:1249–56.
de Lade CG, Andreazzi AE, Bolotari M, Costa VMG, Peters VM, Guerra MO. Effects of moderate intensity endurance training vs. high intensity interval training on weight gain, cardiorespiratory capacity, and metabolic profile in postnatal overfed rats. Diabetol Metab Syndr. 2018;10:70.
Moreira VM, Almeida D, da Silva Franco CC, Gomes RM, Palma-Rigo K, Prates KV, et al. Moderate exercise training since adolescence reduces Walker 256 tumour growth in adult rats. J Physiol. 2019;597:3905–25.
Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148:5339–47.
Scomparin DX, Gomes RM, Grassiolli S, Rinaldi W, Martins AG, de Oliveira JC, et al. Autonomic activity and glycemic homeostasis are maintained by precocious and low intensity training exercises in MSG-programmed obese mice. Endocrine. 2009;36:510–7.
Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969;22:158–61.
Hermans MP, Schmeer W, Henquin JC. Modulation of the effect of acetylcholine on insulin release by the membrane potential of B cells. Endocrinology. 1987;120:1765–73.
Berry R, Church CD, Gericke MT, Jeffery E, Colman L, Rodeheffer MS. Imaging of adipose tissue. Methods Enzymol. 2014;537:47–73.
Parlee SD, Lentz SI, Mori H, MacDougald OA. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 2014;537:93–122.
Malta A, Souza AA, Ribeiro TA, Francisco FA, Pavanello A, Prates KV, et al. Neonatal treatment with scopolamine butylbromide prevents metabolic dysfunction in male rats. Sci Rep. 2016;6:30745.
Miranda RA, Torrezan R, de Oliveira JC, Barella LF, da Silva Franco CC, Lisboa PC, et al. HPA axis and vagus nervous function are involved in impaired insulin secretion of MSG-obese rats. J Endocrinol. 2016;230:27–38.
Abreu P, Mendes SV, Leal-Cardoso JH, Ceccatto VM. Anaerobic threshold employed on exercise training prescription and performance assessment for laboratory rodents: A short review. Life Sci. 2016;151:1–6.
Ebal E, Cavalie H, Michaux O, Lac G. Effect of a moderate exercise on the regulatory hormones of food intake in rats. Appetite. 2007;49:521–4.
Zhu S, Eclarinal J, Baker MS, Li G, Waterland RA. Developmental programming of energy balance regulation: is physical activity ‘programmable’ than food intake? Proc Nutr Soc. 2015;75:1–5.
Li G, Kohorst JJ, Zhang W, Laritsky E, Kunde-Ramamoorthy G, Baker MS, et al. Early postnatal nutrition determines adult physical activity and energy expenditure in female mice. Diabetes. 2013;62:2773–83.
Liu HW, Mahmood S, Srinivasan M, Smiraglia DJ, Patel MS. Developmental programming in skeletal muscle in response to overnourishment in the immediate postnatal life in rats. J Nutr Biochem. 2013;24:1859–69.
Smorlesi A, Frontini A, Giordano A, Cinti S. The adipose organ: white-brown adipocyte plasticity and metabolic inflammation. Obes Rev,. 2012;13:83–96.
Blessing, W, M Mohammed, and Y Ootsuka, Brown adipose tissue thermogenesis, the basic rest-activity cycle, meal initiation, and bodily homeostasis in rats. Physiol Behav, 2013.
Ootsuka Y, de Menezes RC, Zaretsky DV, Alimoradian A, Hunt J, Stefanidis A, et al. Brown adipose tissue thermogenesis heats brain and body as part of the brain-coordinated ultradian basic rest-activity cycle. Neuroscience. 2009;164:849–61.
Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359.
Rinaldi W, Gomes RM, Scomparin DX, Grassiolli S, Ribeiro TA, Fabricio GS, et al. Low-intensity and moderate exercise training improves autonomic nervous system activity imbalanced by postnatal early overfeeding in rats. J Int Soc Sports Nutr. 2014;11:25.
Morrison SF. Differential regulation of brown adipose and splanchnic sympathetic outflows in rat: roles of raphe and rostral ventrolateral medulla neurons. Clin Exp Pharmacol Physiol. 2001;28:138–43.
Conceicao EP, Moura EG, Oliveira E, Guarda DS, Figueiredo MS, Quitete FT, et al. Dietary calcium supplementation in adult rats reverts brown adipose tissue dysfunction programmed by postnatal early overfeeding. J Nutr Biochem. 2017;39:117–25.
Xiao XQ, Williams SM, Grayson BE, Glavas MM, Cowley MA, Smith MS, et al. Excess weight gain during the early postnatal period is associated with permanent reprogramming of brown adipose tissue adaptive thermogenesis. Endocrinology. 2007;148:4150–9.
Kozak LP, Anunciado-Koza R. UCP1: its involvement and utility in obesity. Int J Obes (Lond). 2008;32:S32–8.
Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY, et al. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1115–25.
Dewal RS, Stanford KI. Effects of exercise on brown and beige adipocytes. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:71–78.
Acknowledgements
The authors would like to thank the technicians, Ms. Maroly Pinto and Mrs. Marli Licero, for working on the care of rats in the Local Animal Facility. The authors would also like to thank the Brazilian federal science agencies: Co-ordination for the Improvement of Higher Education Personnel (CAPES) and National Council for Scientific and Technological Development (CNPq), for the financial support in this work.
Funding
This work was supported by the Co-ordination for the Improvement of Higher Education Personnel (CAPES) and National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Contributions
DLA, KPR, and PCFM designed the study, DLA, VMM, LEC, TAR, CCSF, LPT, MVGR, AROF, MNCP, AP, MDFJ, and RAM conducted the experiments, and analyze the data with RMG, IHT, JAA, and PCFM; DLA wrote the paper, all the authors have read, revised and contributed to the final version of the manuscript. All authors read and approved the final version of the manuscript submitted for publication and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almeida, D.L., Moreira, V.M., Cardoso, L.E. et al. Lean in one way, in obesity another: effects of moderate exercise in brown adipose tissue of early overfed male Wistar rats. Int J Obes 46, 137–143 (2022). https://doi.org/10.1038/s41366-021-00969-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-00969-1