Abstract
Background/objectives
The weight loss following Semaglutide treatment, a GLP-1 receptor agonist, might be responsible for some effects observed on the nonalcoholic fatty liver disease of obese mice.
Subjects/methods
Two groups of C57BL/6 male mice (n = 30/group) were fed the diets Control (C) or high-fat (HF) for 16 weeks. Then, separated into six new groups for an additional four weeks (n = 10/group) and treated with Semaglutide (S, 40 µg/kg) or paired feeding (PF) with S groups (C; C-S; C-PF; HF; HF-S; HF-PF).
Results
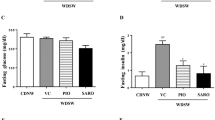
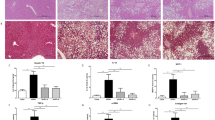
Semaglutide reduced energy consumption leading to weight loss. Simultaneously it improved glucose intolerance, glycated hemoglobin, insulin resistance/sensitivity, plasma lipids, and gastric inhibitory polypeptide. Semaglutide and paired feeding mitigated liver steatosis and adipose differentiation-related protein (Plin2) expression. Semaglutide also improved hormones and adipokines, reduced lipogenesis and inflammation, and increased beta-oxidation. Semaglutide lessened liver glucose uptake and endoplasmic reticulum (ER) stress. Among the 14 genes analyzed, 13 were modified by Semaglutide (93 %, six genes were changed exclusively by Semaglutide, and seven other genes were affected by the combination of Semaglutide and paired feeding). In seven genes, the paired diet showed no effect (50% of the genes tested). No marker was affected exclusively by paired feeding.
Conclusions
Semaglutide and the consequent weight loss reduced obese mice liver inflammation, insulin resistance, and ER stress. However, weight loss alone did show few or no action on some significant study findings, like liver steatosis, leptin, insulin, resistin, and amylin. Furthermore, hepatic inflammation mediated by MCP-1 and partially by TNF-alpha and IL6 were also not reduced by weight loss. Furthermore, weight loss alone did not lessen hepatic lipogenesis as determined by the findings of SREBP-1c, CHREBP, PPAR-alpha, and SIRT1. Semaglutide was implicated in improving glucose uptake and lessening ER stress by reducing GADD45, independent of weight loss.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Christou GA, Katsiki N, Blundell J, Fruhbeck G, Kiortsis DN. Semaglutide as a promising antiobesity drug. Obes Rev. 2019;20:805–15. https://doi.org/10.1111/obr.12839.
Kushner RF, Calanna S, Davies M, Dicker D, Garvey WT, Goldman B, et al. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP trials 1 to 5. Obesity. 2020;28:1050–61. https://doi.org/10.1002/oby.22794.
Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–24. https://doi.org/10.1056/NEJMoa2028395.
Guzman G, Brunt EM, Petrovic LM, Chejfec G, Layden TJ, Cotler SJ. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med. 2008;132:1761–6. https://doi.org/10.5858/132.11.1761.
Lebeaupin C, Vallee D, Hazari Y, Hetz C, Chevet E, Bailly-Maitre B. Endoplasmic reticulum stress signalling and the pathogenesis of nonalcoholic fatty liver disease. J Hepatol. 2018;69:927–47. https://doi.org/10.1016/j.jhep.2018.06.008.
Wei J, Fang D. Endoplasmic reticulum stress signaling and the pathogenesis of hepatocarcinoma. Int J Mol Sci. 2021;22, https://doi.org/10.3390/ijms22041799.
Buratta S, Shimanaka Y, Costanzi E, Ni S, Urbanelli L, Kono N, et al. Lipotoxic stress alters the membrane lipid profile of extracellular vesicles released by Huh-7 hepatocarcinoma cells. Sci Rep. 2021;11:4613 https://doi.org/10.1038/s41598-021-84268-9.
Vianna AB, Aguila MB, Mandarim-de-Lacerda CA. Effects of liraglutide in hypothalamic arcuate nucleus of obese mice. Obesity. 2016;24:626–33. https://doi.org/10.1002/oby.21387.
Vianna AB, Aguila MB, Mandarim-de-Lacerda CA. Beneficial effects of liraglutide (GLP1 analog) in the hippocampal inflammation. Metab Brain Dis. 2017;32:1735–45. https://doi.org/10.1007/s11011-017-0059-4.
Schulte EM, Tuerk PW, Wadden TA, Garvey WT, Weiss D, Hermayer KL, et al. Changes in weight control behaviors and hedonic hunger in a commercial weight management program adapted for individuals with type 2 diabetes. Int J Obes. 2020;44:990–8. https://doi.org/10.1038/s41366-020-0530-x.
Fraulob JC, Ogg-Diamantino R, Santos CF, Aguila MB, Mandarim-de-Lacerda CA. A mouse model of metabolic syndrome: insulin resistance, fatty liver and nonalcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr. 2010;46:212–23. https://doi.org/10.3164/jcbn.09-83.
Aguila MB, Ornellas F, Mandarim-de-Lacerda CA. Nutritional research and fetal programming: parental nutrition influences the structure and function of the organs. Int J Morphol. 2021;39:327–34. https://doi.org/10.4067/s0717-95022021000100327.
Peterson RG, Jackson CV, Zimmerman KM, Alsina-Fernandez J, Michael MD, Emmerson PJ, et al. Glucose dysregulation and response to common anti-diabetic agents in the FATZO/Pco mouse. PLoS One. 2017;12:e0179856 https://doi.org/10.1371/journal.pone.0179856.
Pang J, Xi C, Huang X, Cui J, Gong H, Zhang T. Effects of excess energy intake on glucose and lipid metabolism in C57BL/6 mice. PLoS One. 2016;11:e0146675 https://doi.org/10.1371/journal.pone.0146675.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. https://doi.org/10.1210/jcem.85.7.6661.
Mandarim-de-Lacerda CA. Stereological tools in biomedical research. An Acad Bras Cienc. 2003;75:469–86. https://doi.org/10.1590/S0001-37652003000400006.
Catta-Preta M, Mendonca LS, Fraulob-Aquino J, Aguila MB, Mandarim-de-Lacerda CA. A critical analysis of three quantitative methods of assessment of hepatic steatosis in liver biopsies. Virchows Arch. 2011;459:477–85. https://doi.org/10.1007/s00428-011-1147-1.
Mandarim-de-Lacerda CA, Del-Sol M. Tips for studies with quantitative morphology (morphometry and stereology). Int J Morphol. 2017;35:1482–94. https://doi.org/10.4067/s0717-95022017000401482.
Rao X, Lai D, Huang X. A new method for quantitative real-time polymerase chain reaction data analysis. J Comput Biol. 2013;20:703–11. https://doi.org/10.1089/cmb.2012.0279.
De Minicis S, Day C, Svegliati-Baroni G. From NAFLD to NASH and HCC: pathogenetic mechanisms and therapeutic insights. Curr Pharm Des. 2013;19:5239–49.
Zhong F, Zhou X, Xu J, Gao L. Rodent models of nonalcoholic fatty liver disease. Digestion. 2020;101:522–35. https://doi.org/10.1159/000501851.
Negrin KA, Roth Flach RJ, DiStefano MT, Matevossian A, Friedline RH, Jung D, et al. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLoS One. 2014;9:e107265 https://doi.org/10.1371/journal.pone.0107265.
Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity. 2015;23:2319–20. https://doi.org/10.1002/oby.21358.
Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591–601. https://doi.org/10.1016/j.cmet.2016.02.005.
Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325:1414–25. https://doi.org/10.1001/jama.2021.3224.
Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989 https://doi.org/10.1056/NEJMoa2032183.
Rakipovski G, Rolin B, Nohr J, Klewe I, Frederiksen KS, Augustin R, et al. The GLP-1 Analogs Liraglutide and Semaglutide reduce atherosclerosis in ApoE(−/−) and LDLr(−/−) mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci. 2018;3:844–57. https://doi.org/10.1016/j.jacbts.2018.09.004.
Zhang L, Zhang L, Li L, Holscher C. Semaglutide is neuroprotective and reduces alpha-synuclein levels in the chronic MPTP mouse model of Parkinson’s disease. J Parkinsons Dis. 2019;9:157–71. https://doi.org/10.3233/JPD-181503.
Chubb B, Gupta P, Gupta J, Nuhoho S, Kallenbach K, Orme M. Once-daily oral Semaglutide versus injectable GLP-1 RAs in people with type 2 diabetes inadequately controlled on basal insulin: systematic review and network meta-analysis. Diabetes Ther. 2021;12:1325–39. https://doi.org/10.1007/s13300-021-01034-w.
Li J, He K, Ge J, Li C, Jing Z. Efficacy and safety of the glucagon-like peptide-1 receptor agonist oral semaglutide in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2021;172:108656 https://doi.org/10.1016/j.diabres.2021.108656.
Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102 https://doi.org/10.1016/j.molmet.2020.101102.
Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394:39–50. https://doi.org/10.1016/S0140-6736(19)31271-1.
Barbosa-da-Silva S, Fraulob-Aquino JC, Lopes JR, Mandarim-de-Lacerda CA, Aguila MB. Weight cycling enhances adipose tissue inflammatory responses in male mice. PLoS One. 2012;7:e39837 https://doi.org/10.1371/journal.pone.0039837.
Barbosa-da-Silva S, Silva NC, Aguila MB, Mandarim-de-Lacerda CA. Liver damage is not reversed during the lean period in diet-induced weight cycling in mice. Hepatol Res. 2014;44:450–9. https://doi.org/10.1111/hepr.12138.
Spezani R, Silva RR, Martins FF, Marinho TS, Aguila MB, Mandarim-de-Lacerda CA. Intermittent fasting, adipokines, insulin sensitivity, and hypothalamic neuropeptides in a dietary overload with high-fat or high-fructose diet in mice. J Nutr Biochem. 2020;83:108419 https://doi.org/10.1016/j.jnutbio.2020.108419.
Gonnissen HK, Hulshof T, Westerterp-Plantenga MS. Chronobiology, endocrinology, and energy- and food-reward homeostasis. Obes Rev. 2013;14:405–16. https://doi.org/10.1111/obr.12019.
Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Ronne J, Alanentalo T, Baquero AF, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5, https://doi.org/10.1172/jci.insight.133429.
Adolph TE, Grander C, Grabherr F, Tilg H. Adipokines and nonalcoholic fatty liver disease: multiple interactions. Int J Mol Sci. 2017;18: https://doi.org/10.3390/ijms18081649.
D’Souza AM, Neumann UH, Glavas MM, Kieffer TJ. The glucoregulatory actions of leptin. Mol Metab. 2017;6:1052–65. https://doi.org/10.1016/j.molmet.2017.04.011.
Pretz D, Le Foll C, Rizwan MZ, Lutz TA, Tups A. Hyperleptinemia as a contributing factor for the impairment of glucose intolerance in obesity. FASEB J. 2021;35:e21216 https://doi.org/10.1096/fj.202001147R.
Moonishaa TM, Nanda SK, Shamraj M, Sivaa R, Sivakumar P, Ravichandran K. Evaluation of leptin as a marker of insulin resistance in type 2 diabetes mellitus. Int J Appl Basic Med Res. 2017;7:176–80. https://doi.org/10.4103/ijabmr.IJABMR_278_16.
Schultz A, Da Silva SB, Aguila MB, Mandarim-de-Lacerda CA. Differences and similarities in hepatic lipogenesis, gluconeogenesis and oxidative imbalance in mice fed diets rich in fructose or sucrose. Food Funct. 2015;6:1684–91. https://doi.org/10.1039/c5fo00251f.
Hodson L, Gunn PJ. The regulation of hepatic fatty acid synthesis and partitioning: the effect of nutritional state. Nat Rev Endocrinol. 2019;15:689–700. https://doi.org/10.1038/s41574-019-0256-9.
Libby AE, Bales ES, Monks J, Orlicky DJ, McManaman JL. Perilipin-2 deletion promotes carbohydrate-mediated browning of white adipose tissue at ambient temperature. J Lipid Res. 2018;59:1482–500. https://doi.org/10.1194/jlr.M086249
Marinho TS, Ornellas F, Barbosa-da-Silva S, Mandarim-de-Lacerda CA, Aguila MB. Beneficial effects of intermittent fasting on steatosis and inflammation of the liver in mice fed a high-fat or a high-fructose diet. Nutrition. 2019;65:103–12. https://doi.org/10.1016/j.nut.2019.02.020.
Zhou R, Lin C, Cheng Y, Zhuo X, Li Q, Xu W, et al. liraglutide alleviates hepatic steatosis and liver injury in T2MD rats via a GLP-1R dependent AMPK pathway. Front Pharmacol. 2020;11:600175 https://doi.org/10.3389/fphar.2020.600175.
Iizuka K, Takao K, Yabe D. ChREBP-Mediated regulation of lipid metabolism: involvement of the gut microbiota, liver, and adipose tissue. Front Endocrinol. 2020;11:587189 https://doi.org/10.3389/fendo.2020.587189.
Chen J, Zhao H, Ma X, Zhang Y, Lu S, Wang Y, et al. GLP-1/GLP-1R Signaling in regulation of adipocyte differentiation and lipogenesis. Cell Physiol Biochem. 2017;42:1165–76. https://doi.org/10.1159/000478872.
Scerif M, Goldstone AP, Korbonits M. Ghrelin in obesity and endocrine diseases. Mol Cell Endocrinol. 2011;340:15–25. https://doi.org/10.1016/j.mce.2011.02.011.
Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20:5–21. https://doi.org/10.1111/dom.13129.
Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–26. https://doi.org/10.1089/jir.2008.0027.
Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun. 2010;11:145–56. https://doi.org/10.1159/000289203.
Akash MSH, Rehman K, Liaqat A. Tumor necrosis factor-alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2018;119:105–10. https://doi.org/10.1002/jcb.26174.
Zhou JY, Poudel A, Welchko R, Mekala N, Chandramani-Shivalingappa P, Rosca MG, et al. liraglutide improves insulin sensitivity in high fat diet induced diabetic mice through multiple pathways. Eur J Pharmacol. 2019;861:172594 https://doi.org/10.1016/j.ejphar.2019.172594.
Luo Y, Yang P, Li Z, Luo Y, Shen J, Li R, et al. liraglutide improves nonalcoholic fatty liver disease in diabetic mice by modulating inflammatory signaling pathways. Drug Des Devel Ther. 2019;13:4065–74. https://doi.org/10.2147/DDDT.S224688.
Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015;58:221–32. https://doi.org/10.1007/s00125-014-3451-1.
Deng XQ, Chen LL, Li NX. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007;27:708–15. https://doi.org/10.1111/j.1478-3231.2007.01497.x.
Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–35. https://doi.org/10.1038/nature17041.
Shore GC, Papa FR, Oakes SA. Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol. 2011;23:143–9. https://doi.org/10.1016/j.ceb.2010.11.003.
Li X, Wang Y, Wang H, Huang C, Huang Y, Li J. Endoplasmic reticulum stress is the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in liver fibrosis. Inflamm Res. 2015;64:1–7. https://doi.org/10.1007/s00011-014-0772-y.
Cao J, Dai DL, Yao L, Yu HH, Ning B, Zhang Q, et al. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol Cell Biochem. 2012;364:115–29. https://doi.org/10.1007/s11010-011-1211-9.
Guo X, Tang R, Yang S, Lu Y, Luo J, Liu Z. Rutin and its combination with inulin attenuate gut dysbiosis, the inflammatory status and endoplasmic reticulum stress in Paneth cells of obese mice induced by high-fat diet. Front Microbiol. 2018;9:2651 https://doi.org/10.3389/fmicb.2018.02651.
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. https://doi.org/10.1126/science.1103160.
Santos FO, Correia BRO, Marinho TS, Barbosa-da-Silva S, Mandarim-de-Lacerda CA, Souza-Mello V. Anti-steatotic linagliptin pleiotropic effects encompasses suppression of de novo lipogenesis and ER stress in high-fat-fed mice. Mol Cell Endocrinol. 2020;509:110804 https://doi.org/10.1016/j.mce.2020.110804.
Vanweert F, Boone SC, Brouwers B, Mook-Kanamori DO, de Mutsert R, Rosendaal FR, et al. The effect of physical activity level and exercise training on the association between plasma branched-chain amino acids and intrahepatic lipid content in participants with obesity. Int J Obes. 2021;45:1510–20. https://doi.org/10.1038/s41366-021-00815-4.
Motta VF, Aguila MB, Mandarim-de-Lacerda CA. High-intensity interval training (swimming) significantly improves the adverse metabolism and comorbidities in diet-induced obese mice. J Sports Med Phys Fitness. 2016;56:655–63.
Schultz A, Mendonca LS, Aguila MB, Mandarim-de-Lacerda CA. Swimming training beneficial effects in a mice model of nonalcoholic fatty liver disease. Exp Toxicol Pathol. 2012;64:273–82. https://doi.org/10.1016/j.etp.2010.08.019.
Acknowledgements
The authors are grateful for the technical assistance of Mrs. Aline Penna. The study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil) (CNPq, Grant nos. 302.920/2016-1 and 40.60.81/2018-2 to CML and 305.865/2017-0 to MBA), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (Faperj, Grant nos. E-26/202.935/2017 and E-26/010.100947/2018 to CML and E-26/202.795/2017 to MBA). RPS received a bursary from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001. These foundations had no interference in the accomplishment and submission of the study.
Author information
Authors and Affiliations
Contributions
Conception and design of the study (MBA, CML); acquisition of data, analysis, and interpretation of data (RPS, TSM); drafting the article or revising it critically for valuable intellectual content (RPS, TSM, LMC); final approval of the version to be submitted (MBA, CML).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pontes-da-Silva, R.M., de Souza Marinho, T., de Macedo Cardoso, L.E. et al. Obese mice weight loss role on nonalcoholic fatty liver disease and endoplasmic reticulum stress treated by a GLP-1 receptor agonist. Int J Obes 46, 21–29 (2022). https://doi.org/10.1038/s41366-021-00955-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-00955-7