Abstract
Objective
Prenatal metabolomics profiles, providing measures of in utero nutritional and environmental exposures, may improve the prediction of childhood outcomes. We aimed to identify prenatal plasma metabolites associated with early childhood body mass index (BMI) trajectories and overweight/obesity risk in offspring.
Methods
This study included 450 African American mother-child pairs from the Conditions Affecting Neurocognitive Development and Learning in Early Childhood Study. An untargeted metabolomics analysis was performed on the mothers’ plasma samples collected during the second trimester. The children’s BMI-z-score trajectories from birth to age 4 [rising-high- (9.8%), moderate- (68.2%), and low-BMI (22.0%)] and overweight/obesity status at age 4 were the main outcomes. The least absolute shrinkage and selection operator (LASSO) was used to select the prenatal metabolites associated with childhood outcomes.
Results
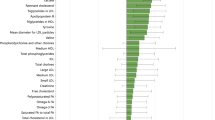
The mothers were 24.5 years old on average at recruitment, 76.4% having education less than 12 years and 80.0% with Medicaid or Medicare. In LASSO, seven and five prenatal metabolites were associated with the BMI-z-score trajectories and overweight/obese at age 4, respectively. These metabolites are mainly from/relevant to the pathways of steroid biosynthesis, amino acid metabolism, vitamin B complex, and xenobiotics metabolism (e.g., caffeine and nicotine). The odds ratios (95% CI) associated with a one SD increase in the prenatal metabolite risk scores (MRSs) constructed from the LASSO-selected metabolites were 2.97 (1.95–4.54) and 2.03 (1.54–2.67) for children being in the rising-high-BMI trajectory group and overweight/obesity at age 4, respectively. The MRSs significantly improved the risk prediction for childhood outcomes beyond traditional prenatal risk factors. The increase (95% CI) in the area under the receiver operating characteristic curves were 0.10 (0.03–0.18) and 0.07 (0.02–0.12) for the rising-high-BMI trajectory (P = 0.005) and overweight/obesity at age 4 (P = 0.007), respectively.
Conclusions
Prenatal metabolomics profiles advanced prediction of early childhood growth trajectories and obesity risk in offspring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2015–2016. National Center for Health Statistics: Health E-Stats; 2018.
Hammond RA, Levine R. The economic impact of obesity in the United States. Diabetes Metab Syndr Obes. 2010;3:285–95.
Cawley J. The economics of childhood obesity. Health Aff (Millwood). 2010;29:364–71.
Trasande L, Chatterjee S. The impact of obesity on health service utilization and costs in childhood. Obesity (Silver Spring). 2009;17:1749–54.
Heindel JJ, Vandenberg LN. Developmental origins of health and disease: a paradigm for understanding disease cause and prevention. Curr Opin Pediatr. 2015;27:248–53.
Hu Z, Tylavsky FA, Han JC, Kocak M, Fowke JH, Davis RL, et al. Maternal metabolic factors during pregnancy predict early childhood growth trajectories and obesity risk: the CANDLE Study. Int J Obes (Lond). 2019;43:1914–22.
Zhu Y, Olsen SF, Mendola P, Yeung EH, Vaag A, Bowers K, et al. Growth and obesity through the first 7 y of life in association with levels of maternal glycemia during pregnancy: a prospective cohort study. Am J Clin Nutr. 2016;103:794–800.
Zhao P, Liu E, Qiao Y, Katzmarzyk PT, Chaput JP, Fogelholm M, et al. Maternal gestational diabetes and childhood obesity at age 9-11: results of a multinational study. Diabetologia. 2016;59:2339–48.
Gaillard R, Steegers EA, Franco OH, Hofman A, Jaddoe VW. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int J Obes (Lond). 2015;39:677–85.
Guo L, Liu J, Ye R, Liu J, Zhuang Z, Ren A. Gestational weight gain and overweight in children aged 3-6 years. J Epidemiol. 2015;25:536–43.
Hu Z, Tylavsky FA, Kocak M, Fowke JH, Han JC, Davis RL, et al. Effects of maternal dietary patterns during pregnancy on early childhood growth trajectories and obesity risk: the CANDLE study. Nutrients. 2020;12:465.
Zhu Y, Olsen SF, Mendola P, Halldorsson TI, Yeung EH, Granstrom C, et al. Maternal dietary intakes of refined grains during pregnancy and growth through the first 7 y of life among children born to women with gestational diabetes. Am J Clin Nutr. 2017;106:96–104.
Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond). 2008;32:201–10.
Riedel C, Schonberger K, Yang S, Koshy G, Chen YC, Gopinath B, et al. Parental smoking and childhood obesity: higher effect estimates for maternal smoking in pregnancy compared with paternal smoking-a meta-analysis. Int J Epidemiol. 2014;43:1593–606.
La Merrill M, Birnbaum LS. Childhood obesity and environmental chemicals. Mt Sinai J Med. 2011;78:22–48.
Gutierrez-Torres DS, Barraza-Villarreal A, Hernandez-Cadena L, Escamilla-Nunez C, Romieu I. Prenatal exposure to endocrine disruptors and cardiometabolic risk in preschoolers: a systematic review based on cohort studies. Ann Glob Health. 2018;84:239–49.
Souza RT, Mayrink J, Leite DF, Costa ML, Calderon IM, Rocha Filho EA, et al. Metabolomics applied to maternal and perinatal health: a review of new frontiers with a translation potential. Clinics (Sao Paulo). 2019;74:e894.
Chen Q, Francis E, Hu G, Chen L. Metabolomic profiling of women with gestational diabetes mellitus and their offspring: Review of metabolomics studies. J Diabetes Complications. 2018;32:512–23.
Maitre L, Fthenou E, Athersuch T, Coen M, Toledano MB, Holmes E, et al. Urinary metabolic profiles in early pregnancy are associated with preterm birth and fetal growth restriction in the Rhea mother-child cohort study. BMC Med. 2014;12:110.
Luthra G, Vuckovic I, Bangdiwala A, Gray H, Redmon JB, Barrett ES, et al. First and second trimester urinary metabolic profiles and fetal growth restriction: an exploratory nested case-control study within the infant development and environment study. BMC Pregnancy Childbirth. 2018;18:48.
Carter RA, Pan K, Harville EW, McRitchie S, Sumner S. Metabolomics to reveal biomarkers and pathways of preterm birth: a systematic review and epidemiologic perspective. Metabolomics. 2019;15:124.
Tylavsky FA, Kocak M, Murphy LE, Graff JC, Palmer FB, Volgyi E, et al. Gestational vitamin 25(OH)D status as a risk factor for receptive language development: a 24-month, longitudinal, observational study. Nutrients. 2015;7:9918–30.
Colon-Ramos U, Racette SB, Ganiban J, Nguyen TG, Kocak M, Carroll KN, et al. Association between dietary patterns during pregnancy and birth size measures in a diverse population in Southern US. Nutrients. 2015;7:1318–32.
Fernandez-Barres S, Romaguera D, Valvi D, Martinez D, Vioque J, Navarrete-Munoz EM, et al. Mediterranean dietary pattern in pregnant women and offspring risk of overweight and abdominal obesity in early childhood: the INMA birth cohort study. Pediatr Obes. 2016;11:491–9.
Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC growth charts for children aged 0-59 months in the United States. MMWR Recomm Rep. 2010;59:1–15.
Aris IM, Chen LW, Tint MT, Pang WW, Soh SE, Saw SM, et al. Body mass index trajectories in the first two years and subsequent childhood cardio-metabolic outcomes: a prospective multi-ethnic Asian cohort study. Sci Rep. 2017;7:8424.
National Center for Health Statistics. Growth Charts. https://www.cdc.gov/growthcharts/index.htm 2010.
Xi B, Gu H, Baniasadi H, Raftery D. Statistical analysis and modeling of mass spectrometry-based metabolomics data. Methods Mol Biol. 2014;1198:333–53.
Bijlsma S, Bobeldijk I, Verheij ER, Ramaker R, Kochhar S, Macdonald IA, et al. Large-scale human metabolomics studies: a strategy for data (pre-) processing and validation. Anal Chem. 2006;78:567–74.
Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc. 1996;58:267–88.
Bartels A, O’Donoghue K. Cholesterol in pregnancy: a review of knowns and unknowns. Obstet Med. 2011;4:147–51.
Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007;92:287–98.
Masuyama H, Mitsui T, Nobumoto E, Hiramatsu Y. The effects of high-fat diet exposure in utero on the obesogenic and diabetogenic traits through epigenetic changes in adiponectin and leptin gene expression for multiple generations in female mice. Endocrinology. 2015;156:2482–91.
McMillen IC, Adam CL, Muhlhausler BS. Early origins of obesity: programming the appetite regulatory system. J Physiol. 2005;565:9–17.
Liu J, Iqbal A, Raslawsky A, Browne RW, Patel MS, Rideout TC. Influence of maternal hypercholesterolemia and phytosterol intervention during gestation and lactation on dyslipidemia and hepatic lipid metabolism in offspring of Syrian golden hamsters. Mol Nutr Food Res. 2016;60:2151–60.
Rideout TC, Movsesian C, Tsai YT, Iqbal A, Raslawsky A, Patel MS. Maternal phytosterol supplementation during pregnancy and lactation modulates lipid and lipoprotein response in offspring of apoE-deficient mice. J Nutr. 2015;145:1728–34.
Ozyurt H, Totan A, Sahin S, Kilinc C, Sogut S, Akyol O. Maternal serum and amniotic fluid hydroxyproline levels in neural tube defects. Fetal Diagn Ther. 2003;18:321–3.
Messia MC, Marconi E. Innovative and rapid procedure for 4-hydroxyproline determination in meat-based foods. Methods Mol Biol. 2012;828:281–9.
Salim D, Abou EI-Roos N. Detection of phosphates and hydroxyproline in some meat products. BVMJ. 2013;3:1–9.
Murrin CM, Heinen MM, Kelleher CC. Are dietary patterns of mothers during pregnancy related to children’s weight status? Evidence from the lifeways cross- generational cohort study. AIMS Public Health. 2015;2:274–96.
Martin CL, Siega-Riz AM, Sotres-Alvarez D, Robinson WR, Daniels JL, Perrin EM, et al. Maternal dietary patterns during pregnancy are associated with child growth in the first 3 years of life. J Nutr. 2016;146:2281–8.
Hayward C, Brock DJ. Fibrillin-1 mutations in Marfan syndrome and other type-1 fibrillinopathies. Hum Mutat. 1997;10:415–23.
Institute of Medicine. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: The National Academies Press; 1998. p. 592.
Horan MK, McGowan CA, Gibney ER, Donnelly JM, McAuliffe FM. The association between maternal dietary micronutrient intake and neonatal anthropometry - secondary analysis from the ROLO study. Nutr J. 2015;14:105.
Muthayya S, Kurpad AV, Duggan CP, Bosch RJ, Dwarkanath P, Mhaskar A, et al. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban South Indians. Eur J Clin Nutr. 2006;60:791–801.
Luo ZC, Fraser WD, Julien P, Deal CL, Audibert F, Smith GN, et al. Tracing the origins of “fetal origins” of adult diseases: programming by oxidative stress? Med Hypotheses. 2006;66:38–44.
Loy SL, Sirajudeen KN, Hamid, Jan JM. The effects of prenatal oxidative stress levels on infant adiposity development during the first year of life. J Dev Orig Health Dis. 2014;5:142–51.
Sved S, Hossie RD, McGilveray IJ. The human metabolism of caffeine to theophylline. Res Commun Chem Pathol Pharmacol. 1976;13:185–92.
Chen LW, Murrin CM, Mehegan J, Kelleher CC, Phillips CM, Cross-Generation Cohort Study for the L. Maternal, but not paternal or grandparental, caffeine intake is associated with childhood obesity and adiposity: The Lifeways Cross-Generation Cohort Study. Am J Clin Nutr. 2019;109:1648–55.
Papadopoulou E, Botton J, Brantsaeter AL, Haugen M, Alexander J, Meltzer HM, et al. Maternal caffeine intake during pregnancy and childhood growth and overweight: results from a large Norwegian prospective observational cohort study. BMJ Open. 2018;8:e018895.
Li DK, Ferber JR, Odouli R. Maternal caffeine intake during pregnancy and risk of obesity in offspring: a prospective cohort study. Int J Obes (Lond). 2015;39:658–64.
Goldstein A, Warren R. Passage of caffeine into human gonadal and fetal tissue. Biochem Pharmacol. 1962;11:166–8.
Xu D, Wu Y, Liu F, Liu YS, Shen L, Lei YY, et al. A hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic programmed alteration in offspring rats of IUGR induced by prenatal caffeine ingestion. Toxicol Appl Pharmacol. 2012;264:395–403.
Xu D, Zhang B, Liang G, Ping J, Kou H, Li X, et al. Caffeine-induced activated glucocorticoid metabolism in the hippocampus causes hypothalamic-pituitary-adrenal axis inhibition in fetal rats. PLoS One. 2012;7:e44497.
Wu YM, Luo HW, Kou H, Wen YX, Shen L, Pei LG, et al. Prenatal caffeine exposure induced a lower level of fetal blood leptin mainly via placental mechanism. Toxicol Appl Pharmacol. 2015;289:109–16.
Rayfield S, Plugge E. Systematic review and meta-analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J Epidemiol Community Health. 2017;71:162–73.
Gao YJ, Holloway AC, Zeng ZH, Lim GE, Petrik JJ, Foster WG, et al. Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obes Res. 2005;13:687–92.
Ino T. Maternal smoking during pregnancy and offspring obesity: meta-analysis. Pediatr Int. 2010;52:94–9.
George JD, Price CJ, Marr MC, Myers CB, Jahnke GD. Evaluation of the developmental toxicity of isoeugenol in Sprague-Dawley (CD) rats. Toxicol Sci. 2001;60:112–20.
National Center for Health Statistics. Prevalence of Obesity and Severe Obesity Among Adults. United States: National Center for Health Statistics; 2017–2018.
Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308.
Benn M, Tybjaerg-Hansen A, McCarthy MI, Jensen GB, Grande P, Nordestgaard BG. Nonfasting glucose, ischemic heart disease, and myocardial infarction: a Mendelian randomization study. J Am Coll Cardiol. 2012;59:2356–65.
Acknowledgements
We thank all the participants of the CANDLE study. This study was supported by the startup fund of QZ from the University of Tennessee Heath Science Center. The CANDLE study was supported by the Urban Child Institute, the University of Tennessee Heath Science Center, and the National Institutes of Health grants (1R01HL109977, 1UG3OD023271-01, and 5R01HL109977-05). QZ was also supported by the grant R01AG061917 from the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
QZ and FAT—designed research; QZ, JL, DK, and FAT—conducted metabolomics data collection; QZ, ZH, and MH—conducted data analysis; KZL, NRB, WAM, and FAT—collected clinical data and biosamples and management of the CANDLE study; QZ and FAT—drafted the manuscript; JHF, JCH, and KZL—provided critical review and revisions of the manuscript; QZ—had primary responsibility for final content; and all authors: reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, Q., Hu, Z., Kocak, M. et al. Associations of prenatal metabolomics profiles with early childhood growth trajectories and obesity risk in African Americans: the CANDLE study. Int J Obes 45, 1439–1447 (2021). https://doi.org/10.1038/s41366-021-00808-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-00808-3
This article is cited by
-
Metabolomic data presents challenges for epidemiological meta-analysis: a case study of childhood body mass index from the ECHO consortium
Metabolomics (2024)
-
Early ascending growth is associated with maternal lipoprotein profile during mid and late pregnancy and in cord blood
International Journal of Obesity (2023)