Abstract
Introduction
Obesity has numerous etiologies and includes biological factors. Studies have demonstrated that the human adenovirus subtype 36 (Adv36) is an adipogenic agent and causes metabolic alterations. Study results on the prevalence of Adv36 and clinical effects in humans vary substantially. This was a systematic review to summarize the studies on the prevalence of Adv36 infection and its association with human obesity.
Methods
A systematic literature review was conducted using the preferred reporting items for systematic reviews and meta-analysis (PRISMA). Observational or experimental studies found in the Medline, Embase, LILACS, Science Direct and SciELO databases that presented results on the prevalence of Adv36 in humans were included.
Results
Thirty-seven studies were screened. A total of 10,300 adults aged 18–70 years and 4585 children and adolescents aged 3–18 years were assessed. The average prevalence of Adv36 among adults was 22.9%, ranging from 5.5% to 49.8%. Among children and adolescents, the average prevalence of Adv36 was 28.9%, ranging from 7.5% to 73.9%. There was a positive statistical relationship between Adv36 and weight gain, obesity, or metabolic changes in 31 studies. However, in four studies there was no association with obesity, and in one, no association was described. One of the studies showed an inverse correlation, i.e., Adv36 was a protective factor against obesity.
Conclusion
Strong evidence suggested a positive association between viral infection and obesity. However, due to the multi-causality of obesity and heterogeneity of studies, diagnostic tests should be standardized and easily accessible by the population to estimate the overall prevalence of Adv36 infection and its association with obesity.
Similar content being viewed by others
Introduction
Obesity is a public health issue [1, 2], and its prevalence has increased significantly in all age groups and practically in all countries [1,2,3,4]. It is directly related to several complications and diseases [5,6,7,8,9] and may result in psychological changes, financial and life expectancy losses [1,2,3, 10,11,12,13]. Its etiology has multiple factors [14,15,16,17,18,19], in addition to the high cost and the high failure rate of different treatment options [16,17,18,19]. However, in many cases, there is no linearity between exposure to the factors involved with the onset of obesity and the effective development of the disease or a precise relationship with the expected metabolic alterations in people with obesity [20,21,22].
With the aim of identifying alternative factors to those intrinsically related to obesity, such as genetics, physical inactivity, and dietary habits [1, 2, 10], several authors have dedicated themselves to the study of biological agents as one of the causal factors for obesity. Since the 1980s, published studies have shown the adipogenic effect and metabolic alterations of some viruses in several animal species [22,23,24,25,26,27,28,29,30,31,32,33,34]. However, there is still doubt about the role of these agents in the current human obesity pandemic. Although some studies have found an association between the infection of an avian adenovirus (SMAM-1) [35] and Adv36 with human obesity and/or metabolic alterations in adults [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] and in children and adolescents [39, 47, 52,53,54,55,56,57,58,59,60,61,62,63,64,65,66], other studies have shown divergent results in specific subject groups or using different diagnostic methods [67,68,69,70,71,72].
Adv36 was the first human virus to be identified as causing obesity in animals [33]. Adv36 infected chickens, mice, and rats gained significantly more weight and adipose tissue than uninfected controls [26, 33, 34]. A longitudinal study on monkeys has shown a 15% increase in body weight and a 29% reduction in serum cholesterol levels after natural infection [31]. Experimental infection of monkeys with Adv36 led to an almost fourfold increase in body weight compared to uninfected controls and a 58% increase in body fat versus controls [31]. Serum cholesterol decreased significantly (p < 0.006) compared to controls [31]. Adv36 is the only virus that was related to obesity and/or metabolic alterations in naturally infected humans [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66] and has been the most studied pathogen when it comes to biological factors causing the human obesity epidemic [73,74,75,76].
Research on Adv36 effects on humans varies considerably with respect to the infection prevalence and consequences to the diverse populations studied. Therefore, a systematic review would provide the best way to examine these results.
The aim of this study was to assess the prevalence of Adv36 infection in humans and examine the effects based on available scientific evidence.
Material and methods
Searching
The search strategy was performed using Medline and EMBASE bibliographic databases and the platforms of LILACS, Ovid, Science Direct, and SCIELO. The reference lists of the review papers were hand-searched to identify further eligible studies. Duplicate publications were checked.
Two investigators conducted independent searches in parallel, and any discrepancy was discussed and resolved by consensus with a third reviewer. The following terms were used: “obesity”; [textword] or “overweight”; [textword] and “ad36” [textword] or “Adv36” [textword] or “HAdV36” [textword] or “adenovirus 36” [textword] or “adenovirus36” [textword] or “human adenovirus 36” [textword] or “adenovirus-36” [textword] using Boolean operators. A filter “study in humans” was used.
Selection
The eligibility criteria were the following: original observational (cross-sectional, cohort, control case) studies or experimental (clinical, quasi-experimental) studies on humans assessing the prevalence of Adv36 infection, published until December 2019, with no language restrictions.
Experimental studies in animals or in vitro, genetic sequencing, literature review, editorials, opinion articles, case reports, and letters to the editor were excluded.
Validity assessment
The eligibility screening was checked by three independent researchers, who read the titles and abstracts of all articles initially selected to identify those that met the inclusion criteria and those that were not promptly excluded. Disagreements were resolved by discussions among researchers until consensus was reached.
Data extraction
A standardized form was designed, pre-tested, reviewed, and used by three independent researchers for data extraction. Disagreements were resolved by discussions among the researchers involved in the study. The selected studies were critically appraised, and the data were recorded in a table that allowed for a comparison between the studies.
Quantitative data synthesis
The characteristics of the studies, such as the year of publication, target population and sample size, study design, and method for estimating Adv36 infection were recorded. Data were collected according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) checklist [77], and a four-phase diagram was developed (Fig. 1). The main information extracted from each included article was as follows: (1) author and year of publication; (2) studied population, site, and period of data collection; (3) study design and sample size; (4) gender and mean age; (5) prevalence, comparison between people with or without obesity, and method used for the diagnosis of Adv36; (6) p-value; (7) main study conclusion.
Results
Flow of included studies
The initial search of databases yielded 3095 potentially eligible studies based on the combined use of the descriptors. After screening the titles and abstracts, in addition to excluding duplicates, the full text was assessed by three researchers. After applying the inclusion and exclusion criteria, a total of 37 articles were selected for final analysis, according to the flowchart shown in Fig. 1.
Study characteristics
Thirty-seven original articles published between 2005 and 2019 were included in this systematic review, six with a case-control design [38, 47, 48, 51, 60, 67], six cohorts [36, 37, 42, 43, 49, 62], one retrospective cohort [69], 19 cross-sectional studies [39, 40, 44, 45, 50, 52,53,54,55,56,57,58, 61, 64, 65, 68, 70,71,72], four quasi-experimental [41, 59, 63, 66], and one with a mixed design (transversal + case-control) [46]. Nineteen studies were conducted exclusively on adults [36,37,38, 40,41,42,43,44,45,46, 48,49,50,51, 67,68,69,70,71], 16 on children and adolescents [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66, 72], and two studies [39, 47] were conducted on both adults and children, and thus were listed in both Table 1 (adults) and Table 2 (children).
The published works were based on studies conducted in different countries as follows: ten in the United States of America [36, 42, 53, 55, 57, 59, 65, 66, 69, 72], five in Turkey [46,47,48, 60, 64], four in Italy [37, 38, 41, 70], four in South Korea [40, 52, 54, 62], three in the Czech Republic [56, 61, 63], three in China [45, 50, 71], two in Sweden [39, 43], and one in each of the following countries: Poland [44], Mexico [58], Finland [49], Chile [51], in addition to one carried out jointly by Belgium and the Netherlands [68]. The geographical distribution of the conducted studies is presented in Fig. 2.
Participants
The sample sizes of the reviewed studies ranged from 21 [70] to 1978 [39] among adults, and from 13 [55] to 1179 [56] among children and adolescents. A total of 10,300 adults aged 18–70 years and 4585 children and adolescents aged 3–18 years were assessed.
With regard to sample size ranges, four studies had fewer than 50 subjects [48, 55, 66, 70], seven had between 50 and 100 subjects [41, 44, 46, 52, 59, 62, 65], five had between 101 and 150 subjects [47, 53, 57, 60, 64], six had between 151 and 250 subjects [37, 38, 45, 51, 58, 63], six had between 251 and 500 subjects [49, 50, 54, 67, 69, 72], four had between 501 and 1000 subjects [36, 40, 68, 71], and five had a sample size above 1000 subjects [39, 42, 43, 56, 61].
Adv36 diagnosis
Regarding the diagnostic methods for Adv36, 21 studies used serum neutralization assay (SNA) [36,37,38, 40,41,42,43,44, 46, 48, 52,53,54,55, 57, 59, 62, 64, 67, 68, 72], 12 studies used enzyme-linked immunosorbent assay (ELISA) [39, 47, 49, 51, 56, 58, 61, 63, 65, 66, 69, 71] (one of which used PCR and serology concomitantly) [39], three studies used polymerase chain reaction assay (PCR) as the only diagnostic test [45, 50, 70], and one study used the microneutralization test for the diagnosis of Adv36 [60].
Adv36 prevalence and association with obesity
The weighted prevalence of Adv36 infection among adults was 22.9%, ranging from 5.5% [68] to 49.8% [71]. Among children and adolescents, the weighted prevalence was 28.9%, ranging from 7.5% [64] to 73.9% [58].
The prevalence of Adv36 among adults ranged from 5.7% [68] to 64.7% [37] (people with obesity or overweight) and from 0% [48] to 51.4% [71] (people with body mass index less than 25 kg/m2). Among children and adolescents, the prevalence ranged from 12.7% [64] to 63.6% [57] (people with obesity or overweight) and from 1.6% [64] to 51.6% [57] (people with normal weight).
There was statistical evidence of an association between Adv36 and body weight, obesity, and metabolic alterations in 31 studies [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66]. However, no association was found between Adv36 and phenomena related to obesity in four studies [67,68,69, 72], and no mention was made in this regard in one study [70]. One of the studies showed an inverse correlation, i.e., Adv36 was a protective factor against obesity [71].
Discussion
Despite having been developed with a reasonable degree of bias control, as described by their authors, the studies presented in this systematic review showed intrinsic characteristics related to the method and focus. These peculiarities revealed the complexity of analyzing the data together. The most evident issues for an accurate analysis of the material were the great variability of study designs and sample sizes, in addition to different methods for classifying obesity and adiposity, or for the diagnosis of Adv36 and related alterations, besides the different age groups included in each survey, even in the same project.
The diagnosis of Adv36 infection can be based on the PCR [34, 78, 79]; virus culture in the affected tissue (nasopharynx or lower airway, blood, urine, feces, or adipose tissue, among others); [80] indirect evidence of infection by antibody detection (serology) [79, 81, 82]. The most commonly used serological tests are the ELISA or SNA. Although less sensitive than the ELISA, the PCR test is quite specific, given that the identification of viral DNA in adipose tissue will show strong evidence of previous Adv36 infection [79]. PCR tests are considered the gold standard for infection diagnosis. ELISA detects and measures anti-Adv36 antibodies in blood samples. It is used as a screening test because it is very sensitive; however, it can deliver false results [39]. If the result is positive, confirmation by PCR is suggested [79]. The SNA is less sensitive than the ELISA, but more specific [79, 81, 82]. The length of time of Adv36 infection may also interfere with the results of different diagnostic tests because antibodies may no longer be detectable after a long period of infection. For that reason, sometimes serological tests, such as ELISA or SNA, are more indicated for children, whereas PCR in adipose tissue will likely be more accurate for adults [83]. These characteristics may explain the variability in prevalence found in different studies. In addition to different diagnostic methods, there are socio-cultural differences because the study samples were collected from different geographical regions.
A study by Atkinson et al. [36] was the first to find evidence in humans of the association between Adv36 and obesity-related alterations in lipid profile. The study was conducted on American volunteers with or without obesity and on twins discordant for the infection with Adv36. There was a prevalence of Adv36-positive serology of 30% among subjects with obesity and 11% among subjects without obesity. They concluded that there was a strong association between Adv36 infection and obesity, in addition to significantly lower cholesterol and triglyceride levels in Adv36-positive individuals.
There was a relatively large time gap between the study published by Atkinson and the one by Trovato and colleagues [37] carried out in Italy in 2009. Both studies had a similar design and the same diagnostic methods. Trovato presented Adv36 positivity as high as 64.7% among subjects with obesity and 32.6% among subjects without obesity.
In the following year, five new works were published. Atkinson et al. [52] surveyed children with obesity in South Korean and found a prevalence rate of 30%, which was identical to that found in their first study in the United States, but without significant metabolic alterations. Gabbert [53] and Na [54] have reported a relationship between Adv36 and childhood obesity, with prevalence rates of 22.4% and 28.6% among subjects with obesity, and 7% and 13.6% among those without obesity, respectively. Trovato [38] studied Italians being monitored for non-alcoholic steatohepatitis (NASH) and found a prevalence of antibodies to Adv36 in 41.3% of the sample, of which 46.5% in patients without NASH and 32.3% in those with NASH. Further, the metabolic profile was better than in those who had antibodies to Adv36 compared to their counterparts.
Broderick [67] presented the first conflicting results to those previously described. He assessed American military personnel and found no relationship between Adv36 infection and obesity or metabolic alterations and, interestingly, he noticed a higher prevalence of antibodies to Adv36 in the group without obesity. It should be noted that obesity is a cause for removal from the military, which gives a great motivation to avoid any weight gain, suggesting that this was a selected population. Goossens [68], who conducted a study on patients from Belgium and the Netherlands, presented significantly divergent data by reporting a much lower prevalence than that of the previous studies (5.7% in the group with obesity and 3.9% in the controls). He found no relationship between Adv36 infection and obesity. An interesting fact was that no viral DNA was identified in the adipose tissue in 31 patients with severe obesity.
Almgren et al. [39] mixed several age groups and presented the first study using ELISA for Adv36 diagnosis. They reported an increase in the prevalence of Adv36 infection, which was around 7% in the 1990s and reached a significant 15–20% increase in the following decade, following the same trend as the growth of obesity. It is important to highlight that, if there is a longitudinal increase in the prevalence of Adv36, due to horizontal transmission, the development of immunoprophylaxis for Adv36 and/or the search for specific treatment should be a priority. Different methods of assaying for Adv36 make comparisons between studies and between countries somewhat difficult. ELISA is at least two times more sensitive than serum neutralization, so there may be a higher prevalence in those countries where the studies were done by serum neutralization. SNA is the most definitive if it is positive since it appears that Adv36 does not cross-react to antibodies to other adenoviruses and vice versa [84].
The analyzed literature shows a strong association of Adv36 with obesity or adiposity and with metabolic alterations, including a differentiated lipid profile and improved blood-glucose levels. Krishnapuram et al [34] demonstrated improved glycemic control in both animals and humans that were infected with Adv36.
Caution should be taken in interpreting the results of the studies that found no positive association, or just a weak association, between Adv36 and obesity because they may have been compromised by insufficient sample size or by obesity measurement bias [67, 69]. It is worth mentioning that Adv36 infection is only one of the possible causes of obesity since it is a multifactorial disorder. A paper by Lessan et al. from Abu Dhabi (not included in this review), reported that the prevalence of antibodies was higher than that found in most other countries and there was no correlation with obesity [84]. We know adenovirus antibodies disappear over time, and it is possible that some of the “negative” subjects, particularly in this country where there is such a high prevalence of Adv36, might actually have been infected in the past but their titer is now below the threshold to be called positive. This could affect the association of Adv36 and obesity since these cases would be misclassified. This is true for studies in all the countries [85].
In the period covered by this systematic review, three meta-analyses were published analyzing the relationship between Adv36 infection and human obesity and its metabolic alterations. In the first [86], the authors assessed ten studies and concluded that infection by Adv36 was associated with obesity. However, they did not find an association between Adv36 and changes in metabolic markers or increased abdominal circumference, which suggests that the infection may be associated with the accumulation of subcutaneous fat, but not with visceral fat. The second meta-analysis included 11 case-control studies, with a total of 2508 subjects with obesity and 3005 controls [85]. The study also identified the association between Adv36 infection and obesity, especially among children. The most recent meta-analysis reviewed 24 studies totaling 10,191 surveyed subjects, and the results showed that Adv36 infection increased the risk for obesity, in addition to increasing the risk for weight gain among adults [87]. However, none of the meta-analyses estimated the prevalence of Adv36 infection in humans, the main objective of the present study.
In 2018, Akheruzzaman et al. [88] conducted a systematic review of articles published in the last 25 years, since the first study that linked adenovirus with animal obesity [28]. They evaluated the different types of studies, including biochemical and cellular aspects. All works that involved human beings and assessed research evidence of contact with Adv36 and its association with adiposity or different mechanisms (hormones, metabolism, and cell physiology) were included in their systematic review. They found that most observational studies in humans, regardless of age, linked Adv36 infection to obesity and improvement in glycemic control.
In the current systematic review, we found studies conducted in several countries, with different age groups and focuses, most presenting results similar to those of the study by Atkinson [36]. However, they showed a significant variation for metabolic markers and even pronounced differences in the prevalence of Adv36. However, these studies had great heterogeneity in relation to the design, method of Adv36 diagnosis, age groups even in the same project, and, mainly, sample size.
In an article published in 2000, Hill [14] drew attention to the fact that, despite WHO having defined obesity as the major unaddressed global public health problem, affecting a large part of the population, there was still not enough commitment of policymakers and public health agents to consider this as a real threat to the health of Americans. At that time, obesity was theoretically considered a genetic-environmental problem, and the authors reasoned there should be an effort to change the environmental factors to overcome the problem. With the evidence that Adv36 is a very likely cause of obesity in humans, research into the development of vaccines to prevent Adv36-induced obesity and antiviral drugs to treat infected individuals has not been a priority and represents an unmet need.
Obesity leads to various health problems and poses restrictions and complications to the patient, including premature death [2, 4]. Recent literature shows different levels of evidence that Adv36 affects adiposity and causes metabolic alterations [26, 29,30,31,32,33, 76, 89,90,91,92,93,94,95].
Although experimental infection of animals has facilitated the understanding of the adipogenic effects of Adv36, intentional human exposure to the virus is not possible due to ethical restraints. Therefore, the evidence for Adv36 as an etiology of human obesity depends basically on observational studies.
Given the multifactorial condition of human obesity, it is hard to control bias when measuring the impact of each factor. In the reviewed studies, there was a high prevalence of Adv36 in all age groups, which exceeded 64% among adults and 73% among children and adolescents with obesity and/or metabolic disorders. The vast majority of the reviewed studies have shown a statistically significant association between Adv36 and obesity, adiposity, and related alterations, whereas only a few studies presented divergent results.
Research design, objectives, diagnostic methods, and sample sizes presented great variation in the reviewed studies, which prevented us from carrying out a meta-analysis that would include all the works published so far.
With regard to small samples, it should be noted that the cost is high to carry out the tests, which restricts research to small sample sizes. A major problem is that rapid, inexpensive, and reliable tests, such as the ELISA, are not commercially available. Since Adv36 potentially may be responsible for a significant percentage of human obesity, investigators, governments, foundations, and others should make developing better assays, vaccines, and antiviral drugs a priority in the fight against obesity.
References
Obesity: preventing and managing the global epidemic. Report of a WHO Consultation (WHO Technical Report Series 894). Authors: World Health Organization. 252. Publication date: 2000.
WHO. Obesity and overweight. Updated 16 Feb 2018. Available at: http://www.who.int/news-room/factsheets/detail/obesity-and-overweight. Accessed 5 Jan 2020.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis. Lancet. 2014; 384:766–81. https://doi.org/10.1016/S0140-6736(14)60460-8.
Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33:673–89. https://doi.org/10.1007/s40273-014-0243-x.
Hainer V, Zamrazilová H, Kunešová M, Bendlová B, Aldhoon-Hainerová I. Obesity and infection: reciprocal causality. Physiol Res. 2015;64:S105–19. https://doi.org/10.33549/physiolres.933130.
Mullen JT, Moorman DW, Davenport DL. The obesity paradox: body mass index and outcomes in patients undergoing non-bariatric general surgery. Ann Surg. 2009;250:166–72. https://doi.org/10.1097/SLA.0b013e3181ad8935.
Morgan OW, Bramley A, Fowlkes A, Freedman DS, Taylor TH, Gargiullo P. et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza a(h1n1) disease. PLoS ONE. 2010;5:e9694 https://doi.org/10.1371/journal.pone.0009694.
Arslan E, Atilgan H, Yavasoglu I. The prevalence of Helicobacter pylori in obese subjects. Eur J Intern Med. 2009;20:695–7. https://doi.org/10.1016/j.ejim.2009.07.013.
Uberos J, Molina-Carballo A, Fernandez-Puentes V, Rodriguez-Belmonte R, Munoz-Hoyos A. Overweight and obesity as risk factors for the asymptomatic carrier state of Neisseria meningitidis among a paediatric population. Eur J Clin Microbiol Infect Dis. 2010;29:333–4. https://doi.org/10.1007/s10096-009-0849-7.
Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes. 2013;37:333–40. https://doi.org/10.1038/ijo.2012.62.
Nadeau KJ, Maahs DM, Daniels SR, Eckel RH. Childhood obesity and cardiovascular disease: links and prevention strategies. Nat Rev Cardiol. 2011;8:513–25. https://doi.org/10.1038/nrcardio.2011.86.
Jubber AS. Respiratory complications of obesity. Int J Clin Pract. 2004;58:573–80. https://doi.org/10.1111/j.1368-5031.2004.00166.x.
Roswall N, Li Y, Sandin S, Ström P, Adami HO, Weiderpass E. Changes in body mass index and waist circumference and concurrent mortality among Swedish women. Obesity. 2017;25:215–22. https://doi.org/10.1002/oby.21675.
Hill JO, Wyatt HR, Melanson EL. Genetic and environmental contributions to obesity. Med Clin North Am. 2000;84:333–45. https://doi.org/10.1016/s0025-7125(05)70224-8.
Campbell AMLV. Genetics of obesity. Aust Fam Phys. 2017;46:456–9.
Aveyard P, Lewis A, Tearne S, Hood K, Christian-Brown A, Adab P. et al. Screening and brief intervention for obesity in primary care: a parallel, two-arm, randomized trial. Lancet. 2016;356:2492–500. https://doi.org/10.1016/S0140-6736(16)31893-1.
Zhang Y, Liu JU, Yao J, Ji G, Qian L, Wang J. et al. Obesity: pathophysiology and Intervention. Nutrients.2014;6:5153–83. https://doi.org/10.3390/nu6115153.
English WJ, Williams DB. Metabolic and bariatric surgery: an effective treatment option for obesity and cardiovascular disease. Prog Cardiovasc Dis. 2018;61:253–69. https://doi.org/10.1016/j.pcad.2018.06.003.
Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab Syndr Relat Disord. 2015;13:423–44. https://doi.org/10.1089/met.2015.0095.
Dhurandhar NV. Is obesity caused by an adenovirus?. Expert Rev Anti-Infect Ther. 2012;10:521–4. https://doi.org/10.1586/eri.12.41.
Kim JY, Van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Investig. 2007;117:2621–37. https://doi.org/10.1172/JCI31021.
Lyons MJ, Faust IM, Hemmes RB, Buskirk DR, Hirsch J, Zabriskie JB. A virally induced obesity syndrome in mice. Science. 1982;216:82–5. https://doi.org/10.1126/science.7038878.
Verlaeten O, Griffond B, Khuth ST, Giraudon P, Akaoka H, Belin MF. et al. Down regulation of melanin concentrating hormone in virally induced obesity. Mol Cell Endocrinol. 2001;181:207–19. https://doi.org/10.1016/s0303-7207(01)00488-9.
Herden C, Herzog S, Richt JA, Nesseler A, Christ M, Failing K. et al. Distribution of borna disease virus in the brain of rats infected with an obesity-inducing virus strain. Brain Pathology. 2000;10:39–48. https://doi.org/10.1111/j.1750-3639.2000.tb00241.x.
So PW, Herlihy AH, Bell JD. Adiposity induced by adenovirus 5 inoculations. Int J Obes. 2005;29:603–6. https://doi.org/10.1038/sj.ijo.0802917.
Pasarica M, Shin AC, Yu M, Ou Yang HM, Rathod M, Jen KL. et al. Human adenovirus 36 induces adiposity, increases insulin sensitivity, and alters hypothalamic monoamines in rats. Obesity. 2006;14:1905–13. https://doi.org/10.1038/oby.2006.222.
Carter JK, Ow CL, Smith RE. Rous-associated virus type 7 induces a syndrome in chickens characterized by stunting and obesity. Infect Immunol. 1983;39:410–22.
Dhurandhar NV, Kulkarni P, Ajinkya SM, Sherikar A. Effect of adenovirus infection on adiposity in chicken. Vet Microbiol. 1992;31:101–7. https://doi.org/10.1016/0378-1135(92)90068-5.
Dhurandhar NV, Israel BA, Kolesar JM, Mayhew G, Cook ME, Atkinson RL. Transmissibility of adenovirus-induced adiposity in a chicken model. Int J Obes Relat Metab Disord. 2001;25:990–6. https://doi.org/10.1038/sj.ijo.0801668.
Whigham LD, Israel BA, Atkinson RL. Adipogenic potential of multiple human adenoviruses in vivo and in vitro in animals. Am J Physiol Regul Integr Comput Physiol. 2006;290:R190–4. https://doi.org/10.1152/ajpregu.00479.2005.
Dhurandhar NV, Whigham LD, Abbott DH, Schultz-Darken NJ, Israel BA, Bradley SM. et al. Human adenovirus ad-36 promotes weight gain in male rhesus and marmoset monkeys. J Nutr. 2002;132:3155–60. https://doi.org/10.1093/jn/131.10.3155.
Kapila M, Khosla P, Dhurandhar NV. Novel short-term effects of adenovirus ad-36 on hamster lipoproteins. Int J Obes Relat Metab Disord. 2004;28:1521–7. https://doi.org/10.1038/sj.ijo.0802710.
Dhurandhar NV, Israel BA, Kolesar JM, Mayhew GF, Cook ME, Atkinson RL. Increased adiposity in animals due to a human virus. Int J Obes Relat Metab Disord. 2000;24:989–96. https://doi.org/10.1038/sj.ijo.0801319.
Krishnapuram R, Dhurandhar EJ, Dubuisson O, Kirk-Ballard H, Bajpeyi S, Butte N, et al. Template to improve glycemic control without reducing adiposity or dietary fat. Am J Physiol Endocrinol Metab. 2011;300:E779–89. https://doi.org/10.1152/ajpendo.00703.2010. May
Dhurandhar NV, Kulkarni PR, Ajinkya SM, Sherikar AA, Atkinson RL. Association of adenovirus infection with human obesity. Obes Res. 1997;5:464–9. https://doi.org/10.1002/j.1550-8528.1997.tb00672.x.
Atkinson RL, Dhurandhar NV, Allison DB, Bowen RL, Israel BA, Albu JB. et al. Human adenovirus‐36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes. 2005;29:281–6. https://doi.org/10.1038/sj.ijo.0802830.
Trovato GM, Castro A, Tonzuso A, Garozzo A, Martines GF, Pirri C. et al. Human obesity relationship with Ad36 adenovirus and insulin resistance. Int J Obes. 2009;33:1402–9. https://doi.org/10.1038/ijo.2009.196.
Trovato GM, Martines GF, Garozzo A, Tonzudo A, Timpanaro R, Pirri C. et al. Ad36 adipogenic adenovirus in human non‐alcoholic fatty liver disease. Liver Int. 2010;30:184–90. https://doi.org/10.1111/j.1478-3231.2009.02127.x.
Almgren M, Atkinson R, He J, Hilding A, Hagman E, Wolk A. et al. Adenovirus‐36 is associated with obesity in children and adults in Sweden as determined by rapid ELISA. PLoS ONE. 2012;7:e41652 https://doi.org/10.1371/journal.pone.0041652.
Na HN, Kim J, Lee HS, Shim KW, Kimm H, Jee SH. et al. Association of human adenovirus-36 in overweight Korean adults. Int J Obes. 2012;36:281–5. https://doi.org/10.1038/ijo.2011.102.
Trovato GM, Martines GF, Trovato FM, Pirri C, Pace P, Garozzo A. et al. Adenovirus‐36 seropositivity enhances effects of nutritional intervention on obesity, bright liver, and insulin resistance. Dig Dis Sci. 2012;57:535–44. https://doi.org/10.1007/s10620-011-1903-8.
Lin WY, Dubuisson O, Rubicz R, Liu N, Allison DB, Curran JE. et al. Long‐term changes in adiposity and glycemic control are associated with past adenovirus infection. Diabetes Care. 2013;36:701–7. https://doi.org/10.2337/dc12-1089.
Almgren M, Atkinson RL, Hilding A, He J, Brismar K, Schalling M. et al. Human adenovirus-36 is uncommon in type 2 diabetes and is associated with increased insulin sensitivity in adults in Sweden. Ann Med. 2014;46:539–46. https://doi.org/10.3109/07853890.2014.935469.
Bil-Lula I, Stapor S, Sochocka M, Wolyniec M, Zatonska K, Ilow R. et al. Infectobesity in the polish population—evaluation of an association between adenoviruses type 5, 31, 36 and human obesity. Int J Virol Mol Biol. 2014;3:1–8. https://doi.org/10.5923/j.ijvmb.20140301.01.
Jiao Y, Mao X, Chang X, Abudureyimu K, Zhang C, Lu J. et al. Adenovirus 36 infection expresses cellular APMI and Visfatin genes in overweight Uygur individuals. Diagn Pathol. 2014;9:83–9. https://doi.org/10.1186/1746-1596-9-83.
Ergin S, Altan E, Pilanci O, Sirekbasan S, Cortuk O, Cizmecigil U. et al. The role of adenovirus 36 as a risk factor in obesity: the first clinical study made in the fatty tissues of adults in Turkey. Microb Pathog. 2015;80:57–62. https://doi.org/10.1016/j.micpath.2015.02.008.
Karamese M, Altoparlak U, Turgut A, Aydogdu S, Karamese SA. The relationship between adenovirus‐36 seropositivity, obesity and metabolic profile in Turkish children and adults. Epidemiol Infect. 2015;143:3550–6. https://doi.org/10.1017/S0950268815000679.
Kocazeybek B, Saribas S, Ergin S. The role of Ad-36 as a risk factor in males with gynecomastia. Med Hypotheses. 2015;85:992–6. https://doi.org/10.1016/j.mehy.2015.08.020.
Sabin MA, Burgner D, Atkinson RL, Pei-Lun LZ, Magnussen CG, Cheung M. et al. Longitudinal investigation of adenovirus 36 seropositivity and human obesity: the cardiovascular risk in young finns study. Int J Obes. 2015;39:1644–50. https://doi.org/10.1038/ijo.2015.108.
Waye MM, Chan JC, Tong PC, Ma R, Chan PK. Association of human adenovirus-36 with diabetes, adiposity, and dyslipidaemia in Hong Kong Chinese. Hong Kong Med J. 2015;21:45–7.
Sapunar J, Fonseca L, Molina, Ortiz E, Barra MI, Reimer C. et al. Adenovirus 36 seropositivity is related to obesity risk, glycemic control, and leptin levels in Chilean subjects. Int J Obes. 2020;44:159–66. https://doi.org/10.1038/s41366-019-0321-4.
Atkinson RL, Lee I, Shin HJ, He J. Human adenovirus-36 antibody status is associated with obesity in children. Int J Pediatr Obes. 2010;5:157–60. https://doi.org/10.3109/17477160903111789.
Gabbert C, Donohue M, Arnold J, Schwimmer JB. Adenovirus 36 and obesity in children and adolescents. Pediatrics. 2010;126:721–6. https://doi.org/10.1542/peds.2009-3362.
Na HN, Hong YM, Kim J, Kim HK, Jo I, Nam JH. Association between human adenovirus-36 and lipid disorders in Korean school children. Int J Obes. 2010;34:89–93. https://doi.org/10.1038/ijo.2009.207.
Tosh AK, Broy-Aschenbrenner A, El Khatib J, Ge B. Adenovirus-36 antibody status & BMI comparison among obese Missouri adolescents. Mol Med. 2012;109:402–3. PMCID: PMC6179760
Aldhoon-Hainerova I, Zamrazilova H, Atkinson RL, Dušátková L, Sedláčková B, Hlavatý P. et al. Clinical and laboratory characteristics of 1179 Czech adolescents evaluated for antibodies to human adenovirus 36. Int J Obes. 2014;38:285–91. https://doi.org/10.1038/ijo.2013.72.
Laing EM, Tripp RA, Pollock NK, Baile CA, Della-Fera MA, Rayalam S. et al. Adenovirus 36, adiposity, and bone strength in late-adolescent females. J Bone Miner Res. 2013;28:489–96. https://doi.org/10.1002/jbmr.1776.
Parra-Rojas I, Del Moral-Hernandez O, Salgado-Bernabe AB, Guzman-Guzman IP, Salgado-Goytia L, Munoz-Valle JF. Adenovirus 36 seropositivity and its relation with obesity and metabolic profile in children. Int J Endocrinol. 2013;2013:463194 https://doi.org/10.1155/2013/463194.
Vander Wal JS, Huelsing J, Dubuisson O, Dhurandhar NV. An observational study of the association between adenovirus 36 antibody status and weight loss among youth. Obes Facts. 2013;6:269–78. https://doi.org/10.1159/000353109.
Cakmakliogullari EK, Sanlidag T, Ersoy B, Akcali S, Var A, Cicek C. Are human adenovirus-5 and 36 associated with obesity in children?. J Investig Med. 2014;62:821–4. https://doi.org/10.2310/JIM.0000000000000084.
Dusatkova L, Zamrazilova H, Aldhoon-Hainerova I, Atkinson RL, Sedlackova B, Lee ZP, et al. Association of adenovirus 36 infection with obesity-related gene variants in adolescents. Physiol Res 2015;64:S197–S202. https://doi.org/10.33549/physiolres.933131. Suppl 2
Park S, Kim J, Shin HJ, Hong YM, Sheen YH, Park HL. et al. Tracking study about adenovirus 36 infection: increase of adiposity. J Microbiol Biotechnol. 2015;25:2169–72. https://doi.org/10.4014/jmb.1509.09003.
Zamrazilová H, Aldhoon-Hainerová I, Atkinson RL, Dušátková L, Sedláčková B, Lee ZP. et al. Adenovirus 36 infection: a role in dietary intake and response to inpatient weight management in obese girls. Int J Obes. 2015;39:1757–60. https://doi.org/10.1038/ijo.2015.167.
Kocazeybek B, Dinc HO, Ergin S. Evaluation of adenovirus-36 (Ad-36) antibody seropositivity and adipokine levels in obese children. Microb Pathog. 2017;108:27–31. https://doi.org/10.1016/j.micpath.2017.04.034.
Tosh AK, Wasserman MG, McLeay li MT, Tepe SK. Human adenovirus-36 seropositivity and obesity among Midwestern US adolescents. Int J Adolesc Med Health. 2017. https://doi.org/10.1515/ijamh-2017-0126.
Lavoy EC, Arlinghaus KR, Rooney BV, Gupta P, Atkinson R, Johnston CA. High adenovirus 36 seroprevalence among a population of Hispanic American youth. Int J Adolesc Med Health. 2018. https://doi.org/10.1515/ijamh-2018-0110.
Broderick MP, Hansen CJ, Irvine M, Metzgar D, Campbell K, Baker C. et al. Adenovirus 36 seropositivity is strongly associated with race and gender, but not obesity, among US military personnel. Int J Obes. 2010;34:302–8. https://doi.org/10.1038/ijo.2009.224.
Goossens VJ, de Jager SA, Grauls GE, Gielen M, Vlietinck RF, Derom CA. et al. Lack of evidence for the role of human adenovirus-36 in obesity in a European cohort. Obesity. 2011;19:220–1. https://doi.org/10.1038/oby.2009.452.
Voss JD, Burnett DG, Olsen CH, Haverkos HW, Atkinson RL. Adenovirus 36 Antibodies associated with clinical diagnosis of overweight/obesity but not BMI gain: a military cohort study. J Clin Endocrinol Metab.2014;99:E1708–12. https://doi.org/10.1210/jc.2014-1863.
Ponterio E, Cangemi R, Mariani S, Casella G, De Cesare A, Trovato FM. et al. Adenovirus 36 DNA in human adipose tissue. Int J Obes. 2015;39:1761–4.https://doi.org/10.3390/v7072787.
Zhou Y, Pan Q, Wang X, Zhang L, Xiao F, Guo L. The relationship between human adenovirus 36 and obesity in Chinese Han population. Biosci Rep. 2018; 38. https://doi.org/10.1042/BSR20180553.
Berger PK, Pollock NK, Laing EM, Warden SJ, Hill-Gallant KM, Hausman DB. et al. Association of adenovirus 36 infection with adiposity and inflammatory-related markers in children. J Clin Endocrinol Metab. 2014;99:3240–6. https://doi.org/10.1210/jc.2014-1780.
Dhurandhar NV. A framework for identification of infections that contribute to human obesity. Lancet Infect. Dis. 2011;11:963–69.https://doi.org/10.1016/S1473-3099(11)70274-2.
Hegde V, Dhurandhar NV. Microbes and obesity: interrelationship between infection, adipose tissue and the immune system. Clin Microbiol Infect. 2013;19:314–20.https://doi.org/10.1111/1469-0691.12157.
Rathod MA, Rogers PM, Vangipuram SD, McAllister EJ, Dhurandhar NV. Adipogenic cascade can be induced without adipogenic media by a human adenovirus. Obesity. 2009;17:657–64.https://doi.org/10.1038/oby.2008.630.
Ponterio E, Gnessi L. Adenovirus 36 and obesity: an overview. Viruses. 2015;7:3719–40. https://doi.org/10.3390/v7072787
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1 https://doi.org/10.1186/2046-4053-4-1
Kolesar JM, Miller JA, Dhurandhar NV, Atkinson RL. Direct quantification of AD36 adenovirus DNA by capillary electrophoresis with laser-induced fluorescence. J Chromatogr B Biomed Sci Appl. 2000;744:1–8.
Yeung R, Eshaghi A, Lombos E, Blair J, Mazzulli T, Burton L, et al. Characterization of culture-positive adenovirus serotypes from respiratory specimens in Toronto, Ontario, Canada: September 2007–June 2008. Virol J. 2009;6:11.
Chappell CL, Dickerson M, Day RS, Dubuisson O, Dhurandhar NV. Adenovirus 36 antibody detection: Improving the standard serum neutralization assay. J Virol Methods. 2017;239:69–74.
Dubuisson O, Day RS, Dhurandhar NV. Accurate identification of neutralizing antibodies to adenovirus Ad36—a putative contributor of obesity in humans. J Diabet Compl. 2015;29:83–7.
Nam JH, Na HN, Atkinson RL, Dhurandhar NV. Genomic stability of adipogenic human adenovirus 36. Int J Obes. 2014;38:321–4.
Atkinson RL. Adenovirus and obesity. Chapter 9. In. Haslam DW, Sharma AM, Roux CW. Controversies in obesity. London: Springer-Verlag, 2014. p.75–78.
Lessan N, Saradalekshmi KR, Alkaf B, Majeed M, Barakat MT, Lee ZPL. et al. Obesity and diabetes in an arab population: role of adenovirus 36 infection. Sci Rep. 2020;10:8107https://doi.org/10.1038/s41598-020-65008-x.
Yamada T, Hara K, Kadowaki T. Association of adenovirus 36 infection with obesity and metabolic markers in humans: a meta-analysis of observational studies. PLoS ONE. 2012;7:e42031https://doi.org/10.1371/journal.pone.0042031.
Shang Q, Wang H, Song Y, Wei L, Lavebratt C, Zhang F. et al. Serological data analyses show that adenovirus 36 infection is associated with obesity: a meta-analysis involving 5739 subjects. Obesity. 2014;22:895–900.https://doi.org/10.1002/oby.20533.
Xu MY, Cao B, Wang DF, Guo JH, Chen KL, Shi M. et al. Human adenovirus 36 infection increased the risk of obesity: a meta-analysis update. Medicine. 2015;94:e2357https://doi.org/10.1097/MD.0000000000002357.
Akheruzzaman M, Hegde V, Dhurandhar NV. Twenty-five years of research about adipogenic adenoviruses: a systematic review. Obes Rev. 2019;20:499–509.https://doi.org/10.1111/obr.12808. Apr.
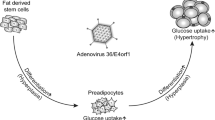
Rogers PM, Fusinski KA, Rathod MA, Loiler SA, Pasarica M, Shaw MK. et al. Human adenovirus Ad-36 induces adipogenesis via its E4 orf-1 gene. Int J Obes. 2008;32:397–406.https://doi.org/10.1038/sj.ijo.0803748.
Thai M, Graham NA, Braas D, Nehil M, Komisopoulou E, Kurdistani SK. et al. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014;19:694–701.https://doi.org/10.1016/j.cmet.2014.03.009.
Dhurandhar NV. Insulin sparing action of adenovirus 36 and its E4orf1 protein. J Diabet Compl. 2013;27:191–9.https://doi.org/10.1016/j.jdiacomp.2012.09.006.
Kusminski CM, Gallardo-Montejano VI, Wang ZV, Hegde V, Bickel PE, Dhurandhar NV. et al. E4orf1 induction in adipose tissue promotes insulin-independent signaling in the adipocyte. Mol Metab. 2015;4:653–64.https://doi.org/10.1016/j.molmet.2015.07.004.
Vangipuram SD, Yu M, Tian J, Stanhope KL, Pasarica M, Havel PJ. et al. Adipogenic human adenovirus-36 reduces leptin expression and secretion and increases glucose uptake by fat cells. Int J Obes. 2007;31:87–96.https://doi.org/10.1038/sj.ijo.0803366.
Rogers PM, Mashtalir N, Rathod MA, Dubuisson O, Wang Z, Dasuri K. et al. Metabolically favorable remodeling of human adipose tissue by human adenovirus type 36. Diabetes. 2008;57:2321–31.https://doi.org/10.2337/db07-1311.
Pasarica M, Mashtalir N, McAllister EJ, Kilroy GE, Koska J, Permana P. et al. Adipogenic human adenovirus ad-36 induces commitment, differentiation, and lipid accumulation in human adipose-derived stem cells. Stem Cells. 2008;26:969–78.https://doi.org/10.1634/stemcells.2007-0868.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva Fernandes, J., Schuelter-Trevisol, F., Cancelier, A.C.L. et al. Adenovirus 36 prevalence and association with human obesity: a systematic review. Int J Obes 45, 1342–1356 (2021). https://doi.org/10.1038/s41366-021-00805-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-00805-6