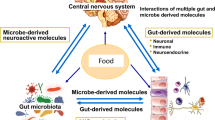
Abstract
The prevalence of obesity is rising every year and associated comorbidities such as cardiovascular diseases are among the leading causes of death worldwide. The gut microbiota has recently emerged as a potential target for therapeutic applications to prevent and treat those comorbidities. In this review, we focus on three conditions related to obesity in which the use of gut microbiota modulators could have benefits; mood disorders, eating behaviors, and body detoxification of persistent organic pollutants (POPs). On one hand, modulation of gut-derived signals to the brain in a context of obesity is involved in the development of neuroinflammation and can subsequently alter behaviors. An altered gut microbiome could change these signals and alleviate their consequences. On the other hand, obesity is associated with an increased accumulation of lipophilic contaminants, such as POPs. Targeting the microbiota could help body detoxication by reducing bioavailability, enhancing degradation by bioremediation or their excretion through the enterohepatic circulation. Thus, a supplementation of prebiotics, probiotics, or synbiotics could represent a complementary strategy to current ones, such as medication and lifestyle modifications, to decrease depression, alter eating behaviors, and lower body burden of pollutants considering the actual obesity epidemic our society is facing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31.
Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214.
Sanchez M, Darimont C, Panahi S, Drapeau V, Marette A, Taylor VH, et al. Effects of a diet-based weight-reducing program with probiotic supplementation on satiety efficiency, eating behaviour traits, and psychosocial behaviours in obese individuals. Nutrients. 2017;9:284.
Zhang L, Nichols RG, Correll J, Murray IA, Tanaka N, Smith PB, et al. Persistent organic pollutants modify gut microbiota-host metabolic homeostasis in mice through aryl hydrocarbon receptor activation. Environ Health Perspect. 2015;123:679–88.
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502.
Anhe FF, Choi BSY, Dyck JRB, Schertzer JD, Marette A. Host-Microbe Interplay in the Cardiometabolic Benefits of Dietary Polyphenols. Trends Endocrinol Metab. 2019;30:384–95.
Masumoto S, Terao A, Yamamoto Y, Mukai T, Miura T, Shoji T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci Rep. 2016;6:31208.
Selma MV, Gonzalez-Sarrias A, Salas-Salvado J, Andres-Lacueva C, Alasalvar C, Orem A, et al. The gut microbiota metabolism of pomegranate or walnut ellagitannins yields two urolithin-metabotypes that correlate with cardiometabolic risk biomarkers: comparison between normoweight, overweight-obesity and metabolic syndrome. Clin Nutr (Edinburgh, Scotland). 2018;37:897–905.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14.
Le Barz M, Anhe FF, Varin TV, Desjardins Y, Levy E, Roy D, et al. Probiotics as complementary treatment for metabolic disorders. Diabetes Metab J. 2015;39:291–303.
Chen Z, Guo L, Zhang Y, Walzem RL, Pendergast JS, Printz RL, et al. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J Clin Invest. 2014;124:3391–406.
Markowiak P, Slizewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:1021.
Schneeberger M, Everard A, Gomez-Valades AG, Matamoros S, Ramirez S, Delzenne NM, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643.
Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2015;65:426–36.
Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exper Mol Med. 2018;50:e450.
Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2016;23:107–13.
Ashrafian F, Shahriary A, Behrouzi A, Moradi HR, Keshavarz Azizi Raftar S, Lari A, et al. Akkermansia muciniphila-Derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in Mice. Front Microbiol. 2019;10:2155.
Cavallari JF, Fullerton MD, Duggan BM, Foley KP, Denou E, Smith BK, et al. Muramyl dipeptide-based postbiotics mitigate obesity-induced insulin resistance via IRF4. Cell Metabol. 2017;25:1063–74e3.
Bailey C, Day C. Metformin: its botanical background. Pract Diabetes Int. 2004;21:115–7.
Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–35.
de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velasquez-Mejia EP, Carmona JA, Abad JM, et al. Metformin Is associated with higher relative abundance of mucin-degrading akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diab Care. 2017;40:54–62.
Adeshirlarijaney A, Zou J, Tran H, Chassaing B, Gewirtz AT. Amelioration of metabolic syndrome by metformin associates with reduced indices of low-grade inflammation independently of the gut microbiota. Am J Physiol Endocrinol Metabol. 2019;317:E1121–E1130.
Allegretti JR, Mullish BH, Kelly C, Fischer M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet. 2019;394:420–31.
Leshem A, Horesh N, Elinav E. Fecal microbial transplantation and its potential application in cardiometabolic syndrome. Front Immunol. 2019;10:1341.
Aron-Wisnewsky J, Clement K, Nieuwdorp M. Fecal microbiota transplantation: a future therapeutic option for obesity/diabetes? Curr Diab Rep. 2019;19:51.
Zhang Z, Mocanu V, Cai C, Dang J, Slater L, Deehan EC, et al. Impact of fecal microbiota transplantation on obesity and metabolic syndrome-a systematic review. Nutrients. 2019;11:2291.
Kootte RS, Levin E, Salojarvi J, Smits LP, Hartstra AV, Udayappan SD, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metabol. 2017;26:611–9e6.
Gundling F, Roggenbrod S, Schleifer S, Sohn M, Schepp W. Patient perception and approval of faecal microbiota transplantation (FMT) as an alternative treatment option for obesity. Obes Sci Pract. 2019;5:68–74.
Cummings DE, Rubino F. Metabolic surgery for the treatment of type 2 diabetes in obese individuals. Diabetologia. 2018;61:257–64.
Anhe FF, Varin TV, Schertzer JD, Marette A. The gut microbiota as a mediator of metabolic benefits after bariatric surgery. Can J Diabetes. 2017;41:439–47.
Liu H, Hu C, Zhang X, Jia W. Role of gut microbiota, bile acids and their cross-talk in the effects of bariatric surgery on obesity and type 2 diabetes. J Diabetes Investig. 2018;9:13–20.
Aron-Wisnewsky J, Dore J, Clement K. The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2012;9:590–8.
van Greevenbroek MM, Schalkwijk CG, Stehouwer CD. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: causes and consequences. Netherl J Med. 2013;71:174–87.
Lizarbe B, Cherix A, Duarte JMN, Cardinaux JR, Gruetter R. High-fat diet consumption alters energy metabolism in the mouse hypothalamus. Int J Obes (2005). 2019;43:1295–304.
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–62.
Waise TMZ, Toshinai K, Naznin F, NamKoong C, Md Moin AS, Sakoda H, et al. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem Biophys Res Commun. 2015;464:1157–62.
Stranahan AM, Hao S, Dey A, Yu X, Baban B. Blood-brain barrier breakdown promotes macrophage infiltration and cognitive impairment in leptin receptor-deficient mice. J Cerebral Blood Flow Metabol. 2016;36:2108–21.
Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–41.
Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019;4:623–32.
Lin P, Ding B, Feng C, Yin S, Zhang T, Qi X, et al. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J Affect Disorders. 2017;207:300–4.
Hassan AM, Mancano G, Kashofer K, Frohlich EE, Matak A, Mayerhofer R, et al. High-fat diet induces depression-like behaviour in mice associated with changes in microbiome, neuropeptide Y, and brain metabolome. Nutr Neurosci. 2019;22:877–93.
Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4:396–403.
Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastroint Liver Physiol. 2012;303:G1288–95.
Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–76.
Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–78.
Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611.
Arentsen T, Qian Y, Gkotzis S, Femenia T, Wang T, Udekwu K, et al. The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol Psych. 2017;22:257–66.
Faith MS, Butryn M, Wadden TA, Fabricatore A, Nguyen AM, Heymsfield SB. Evidence for prospective associations among depression and obesity in population-based studies. Obes Rev. 2011;12:e438–53.
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psych. 2016;21:786–96.
Bruce-Keller AJ, Salbaum JM, Luo M, Et Blanchard, Taylor CM, Welsh DA, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psych. 2015;77:607–15.
Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–94.
Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, et al. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disorders. 2016;202:254–7.
Chen Z, Li J, Gui S, Zhou C, Chen J, Yang C, et al. Comparative metaproteomics analysis shows altered fecal microbiota signatures in patients with major depressive disorder. Neuroreport. 2018;29:417–25.
Kelly JR, Borre Y, C OB, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatric Res. 2016;82:109–18.
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81.
Moloney GM, O’Leary OF, Salvo-Romero E, Desbonnet L, Shanahan F, Dinan TG, et al. Microbial regulation of hippocampal miRNA expression: Implications for transcription of kynurenine pathway enzymes. Behav Brain Res. 2017;334:50–4.
Soto M, Herzog C, Pacheco JA, Fujisaka S, Bullock K, Clish CB, et al. Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol Psych. 2018;23:2287–301.
Gal Z, Huse RJ, Gonda X, Kumar S, Juhasz G, Bagdy G, et al. Anxiety and depression—the role of blood-brain barrier integrity. Neuropsychopharmacologia Hungarica: a Magyar Pszichofarmakologiai Egyesulet lapja = Off J Hungarian Assoc Psychopharmacol. 2019;21:19–25.
Hargrave SL, Davidson TL, Zheng W, Kinzig KP. Western diets induce blood-brain barrier leakage and alter spatial strategies in rats. Behav Neurosci. 2016;130:123–35.
Davidson TL, Monnot A, Neal AU, Martin AA, Horton JJ, Zheng W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiol Behav. 2012;107:26–33.
Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Trans Med. 2014;6:263ra158.
Alcock J, Maley CC, Aktipis CA. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. BioEssays. 2014;36:940–9.
Duca FA, Swartz TD, Sakar Y, Covasa M. Increased oral detection, but decreased intestinal signaling for fats in mice lacking gut microbiota. PLoS ONE. 2012;7:e39748.
Swartz TD, Duca FA, de Wouters T, Sakar Y, Covasa M. Up-regulation of intestinal type 1 taste receptor 3 and sodium glucose luminal transporter-1 expression and increased sucrose intake in mice lacking gut microbiota. British J Nutr. 2012;107:621–30.
Sclafani A, Ackroff K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Reg Integr Comp Physiol. 2012;302:R1119–33.
DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci. 2012;15:1330–5.
Wu C, Garamszegi SP, Xie X, Mash DC. Altered dopamine synaptic markers in postmortem brain of obese subjects. Front Human Neurosci. 2017;11:386.
Delbes AS, Castel J, Denis RGP, Morel C, Quinones M, Everard A, et al. Prebiotics supplementation impact on the reinforcing and motivational aspect of feeding. Front Endocrinol. 2018;9:273.
Nettleton JE, Klancic T, Schick A, Choo AC, Shearer J, Borgland SL, et al. Low-dose stevia (rebaudioside a) consumption perturbs gut microbiota and the mesolimbic dopamine reward system. Nutrients. 2019;11:1248.
Bernard A, Ancel D, Neyrinck AM, Dastugue A, Bindels LB, Delzenne NM, et al. A preventive prebiotic supplementation improves the sweet taste perception in diet-induced obese mice. Nutrients. 2019;11:549.
Alabduljader K, Cliffe M, Sartor F, Papini G, Cox WM, Kubis HP. Ecological momentary assessment of food perceptions and eating behavior using a novel phone application in adults with or without obesity. Eating Behav. 2018;30:35–41.
Rezzi S, Ramadan Z, Martin FP, Fay LB, van Bladeren P, Lindon JC, et al. Human metabolic phenotypes link directly to specific dietary preferences in healthy individuals. J Proteome Res. 2007;6:4469–77.
Sanmiguel CP, Jacobs J, Gupta A, Ju T, Stains J, Coveleskie K, et al. Surgically induced changes in gut microbiome and hedonic eating as related to weight loss: preliminary findings in obese women undergoing bariatric surgery. Psychosomatic Med. 2017;79:880–7.
Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biolog Psych. 2017;82:472–87.
Vaghef-Mehrabany E, Ranjbar F, Asghari-Jafarabadi M, Hosseinpour-Arjmand S, Ebrahimi-Mameghani M. Calorie restriction in combination with prebiotic supplementation in obese women with depression: effects on metabolic and clinical response. Nutr Neurosci. 2019;8:1–15.
Logan AC, Katzman M. Major depressive disorder: probiotics may be an adjuvant therapy. Med Hypoth. 2005;64:533–8.
Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biolog Psych. 2013;74:720–6.
Abildgaard A, Elfving B, Hokland M, Lund S, Wegener G. Probiotic treatment protects against the pro-depressant-like effect of high-fat diet in Flinders Sensitive Line rats. Brain, Behav Immun. 2017;65:33–42.
Osadchiy V, Labus JS, Gupta A, Jacobs J, Ashe-McNalley C, Hsiao EY, et al. Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects. PLoS ONE. 2018;13:e0201772.
Laurans L, Venteclef N, Haddad Y, Chajadine M, Alzaid F, Metghalchi S, et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat Med. 2018;24:1113–20.
Jennis M, Cavanaugh CR, Leo GC, Mabus JR, Lenhard J, Hornby PJ. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol Motil. 2018;30:10.
Tuomainen M, Lindstrom J, Lehtonen M, Auriola S, Pihlajamaki J, Peltonen M, et al. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr Diab. 2018;8:35.
Saez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, Plaza-Diaz J, Gil A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: a review of human clinical trials. Int J Mol Sci. 2016;17:928.
Koppel N, Maini Rekdal V, Balskus EP. Chemical transformation of xenobiotics by the human gut microbiota. Science. 2017;356:eaag2770.
Arrebola JP, Castano A, Esteban M, Bartolome M, Perez-Gomez B, Ramos JJ, et al. Differential contribution of animal and vegetable food items on persistent organic pollutant serum concentrations in Spanish adults. Data from BIOAMBIENT.ES project. Sci Total Environ. 2018;634:235–42.
Pestana D, Faria G, Sa C, Fernandes VC, Teixeira D, Norberto S, et al. Persistent organic pollutant levels in human visceral and subcutaneous adipose tissue in obese individuals–depot differences and dysmetabolism implications. Environ Res. 2014;133:170–7.
Lee DH, Porta M, Jacobs DR Jr., Vandenberg LN. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev. 2014;35:557–601.
Ngwa EN, Kengne AP, Tiedeu-Atogho B, Mofo-Mato EP, Sobngwi E. Persistent organic pollutants as risk factors for type 2 diabetes. Diabetol Metab Syndr. 2015;7:41.
Reaves DK, Ginsburg E, Bang JJ, Fleming JM. Persistent organic pollutants and obesity: are they potential mechanisms for breast cancer promotion? Endocr Relat Cancer. 2015;22:R69–86.
Lim JE, Park SH, Jee SH, Park H. Body concentrations of persistent organic pollutants and prostate cancer: a meta-analysis. Environ Sci Pollut Res Int. 2015;22:11275–84.
Nadal A, Quesada I, Tuduri E, Nogueiras R, Alonso-Magdalena P. Endocrine-disrupting chemicals and the regulation of energy balance. Nat Rev Endocrinol. 2017;13:536–46.
Marushka L, Batal M, David W, Schwartz H, Ing A, Fediuk K, et al. Association between fish consumption, dietary omega-3 fatty acids and persistent organic pollutants intake, and type 2 diabetes in 18 First Nations in Ontario, Canada. Environ Res. 2017;156:725–37.
Lee YM, Kim KS, Kim SA, Hong NS, Lee SJ, Lee DH. Prospective associations between persistent organic pollutants and metabolic syndrome: a nested case-control study. Sci Total Environ. 2014;496:219–25.
Lee YM, Kim KS, Jacobs DR Jr., Lee DH. Persistent organic pollutants in adipose tissue should be considered in obesity research. Obes Rev. 2017;18:129–39.
Gauthier MS, Rabasa-Lhoret R, Prud’homme D, Karelis AD, Geng D, van Bavel B, et al. The metabolically healthy but obese phenotype is associated with lower plasma levels of persistent organic pollutants as compared to the metabolically abnormal obese phenotype. J Clin Endocrinol Metab. 2014;99:E1061–6.
Hue O, Marcotte J, Berrigan F, Simoneau M, Dore J, Marceau P, et al. Increased plasma levels of toxic pollutants accompanying weight loss induced by hypocaloric diet or by bariatric surgery. Obes Surgery. 2006;16:1145–54.
Jansen A, Polder A, Muller MHB, Skjerve E, Aaseth J, Lyche JL. Increased levels of persistent organic pollutants in serum one year after a great weight loss in humans: are the levels exceeding health based guideline values? Sci Total Environ. 2018;622-623:1317–26.
Cheikh Rouhou M, Karelis AD, St-Pierre DH, Lamontagne L. Adverse effects of weight loss: are persistent organic pollutants a potential culprit? Diabetes Metab. 2016;42:215–23.
Pelletier C, Imbeault P, Tremblay A. Energy balance and pollution by organochlorines and polychlorinated biphenyls. Obes Rev. 2003;4:17–24.
Imbeault P, Tremblay A, Simoneau JA, Joanisse DR. Weight loss-induced rise in plasma pollutant is associated with reduced skeletal muscle oxidative capacity. Am J Physiol Endocrinol Metabol. 2002;282:E574–9.
Pelletier C, Doucet E, Imbeault P, Tremblay A. Associations between weight loss-induced changes in plasma organochlorine concentrations, serum T3 concentration, and resting metabolic rate. Toxicol Sci. 2002;67:46–51.
Tremblay A, Pelletier C, Doucet E, Imbeault P. Thermogenesis and weight loss in obese individuals: a primary association with organochlorine pollution. Int J Obes Relat Metab Disord. 2004;28:936–9.
Vizcaino E, Grimalt JO, Fernandez-Somoano A, Tardon A. Transport of persistent organic pollutants across the human placenta. Environ Int. 2014;65:107–15.
Lee MH, Cho ER, Lim JE, Jee SH. Association between serum persistent organic pollutants and DNA methylation in Korean adults. Environ Res. 2017;158:333–41.
Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect. 2008;116:1547–52.
Acharya N, Gautam B, Subbiah S, Rogge MM, Anderson TA, Gao W. Polycyclic aromatic hydrocarbons in breast milk of obese vs normal women: Infant exposure and risk assessment. Sci Total Environ. 2019;668:658–67.
Iszatt N, Janssen S, Lenters V, Dahl C, Stigum H, Knight R, et al. Environmental toxicants in breast milk of Norwegian mothers and gut bacteria composition and metabolites in their infants at 1 month. Microbiome. 2019;7:34.
Wang D, Yan J, Teng M, Yan S, Zhou Z, Zhu W. In utero and lactational exposure to BDE-47 promotes obesity development in mouse offspring fed a high-fat diet: impaired lipid metabolism and intestinal dysbiosis. Arch Toxicol. 2018;92:1847–60.
Chen L, Hu C, Lok-Shun Lai N, Zhang W, Hua J, Lam PKS, et al. Acute exposure to PBDEs at an environmentally realistic concentration causes abrupt changes in the gut microbiota and host health of zebrafish. Environ Pollut. 2018;240:17–26.
Petriello MC, Hoffman JB, Vsevolozhskaya O, Morris AJ, Hennig B. Dioxin-like PCB 126 increases intestinal inflammation and disrupts gut microbiota and metabolic homeostasis. Environ Pollut. 2018;242(Pt A):1022–32.
Zhan J, Liang Y, Liu D, Ma X, Li P, Zhai W, et al. Pectin reduces environmental pollutant-induced obesity in mice through regulating gut microbiota: a case study of p,p’-DDE. Environ Int. 2019;130:104861.
Hoffman JB, Flythe MD, Hennig B. Environmental pollutant-mediated disruption of gut microbial metabolism of the prebiotic inulin. Anaerobe. 2019;55:96–102.
Natividad JM, Agus A, Planchais J, Lamas B, Jarry AC, Martin R, et al. Impaired Aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metabol. 2018;28:737–49 e4.
Petriello MC, Newsome BJ, Dziubla TD, Hilt JZ, Bhattacharyya D, Hennig B. Modulation of persistent organic pollutant toxicity through nutritional intervention: emerging opportunities in biomedicine and environmental remediation. Sci Total Environ. 2014;491-492:11–6.
Arguin H, Sanchez M, Bray GA, Lovejoy JC, Peters JC, Jandacek RJ, et al. Impact of adopting a vegan diet or an olestra supplementation on plasma organochlorine concentrations: results from two pilot studies. Br J Nutr. 2010;103:1433–41.
Girard C, Charette T, Leclerc M, Shapiro BJ, Amyot M. Cooking and co-ingested polyphenols reduce in vitro methylmercury bioaccessibility from fish and may alter exposure in humans. Sci Total Environ. 2018;616-617:863–74.
Ta CA, Zee JA, Desrosiers T, Marin J, Levallois P, Ayotte P, et al. Binding capacity of various fibre to pesticide residues under simulated gastrointestinal conditions. Food Chem Toxicol. 1999;37:1147–51.
Sera N, Morita K, Nagasoe M, Tokieda H, Kitaura T, Tokiwa H. Binding effect of polychlorinated compounds and environmental carcinogens on rice bran fiber. J Nutr Biochem. 2005;16:50–8.
Morita K, Tobiishi K. Increasing effect of nori on the fecal excretion of dioxin by rats. Biosci Biotechnol Biochem. 2002;66:2306–13.
Aislabie JM, Richards NK, Boul HL. Microbial degradation of DDT and its residues - a review. NZ J Agric Res. 2010;40:269–82.
Murinova S, Dercova K, Dudasova H. Degradation of polychlorinated biphenyls (PCBs) by four bacterial isolates obtained from the PCB-contaminated soil and PCB-contaminated sediment. Int Biodet Biodegrad. 2014;91:52–9.
De S, Ghosh S, Dutta SK. Congener specific polychlorinated biphenyl metabolism by human intestinal microbe Clostridium species: Comparison with human liver cell line-HepG2. Ind J Microbiol. 2006;46:199–207.
Lee HS, Lee JC, Lee IK, Moon HB, Chang YS, Jacobs DR Jr., et al. Associations among organochlorine pesticides, Methanobacteriales, and obesity in Korean women. PLoS ONE. 2011;6:e27773.
Jandacek RJ, Tso P. Enterohepatic circulation of organochlorine compounds: a site for nutritional intervention. J Nutr Biochem. 2007;18:163–7.
Fader KA, Nault R, Zhang C, Kumagai K, Harkema JR, Zacharewski TR. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-elicited effects on bile acid homeostasis: alterations in biosynthesis, enterohepatic circulation, and microbial metabolism. Sci Rep. 2017;7:5921.
Wahlstrom A, Sayin SI, Marschall HU, Backhed F. Intestinal crosstalk between Bile Acids and microbiota and its impact on host metabolism. Cell Metabol. 2016;24:41–50.
Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 2014;7:12–8.
Jeun J, Kim S, Cho SY, Jun HJ, Park HJ, Seo JG, et al. Hypocholesterolemic effects of Lactobacillus plantarum KCTC3928 by increased bile acid excretion in C57BL/6 mice. Nutrition. 2010;26:321–30.
Neves AL, Coelho J, Couto L, Leite-Moreira A, Roncon-Albuquerque R Jr. Metabolic endotoxemia: a molecular link between obesity and cardiovascular risk. J Mol Endocrinol. 2013;51:R51–64.
Anhe FF, Roy D, Pilon G, Dudonne S, Matamoros S, Varin TV, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64:872–83.
Skinner MK. Endocrine disruptors in 2015: epigenetic transgenerational inheritance. Nat Rev Endocrinol. 2016;12:68–70.
Krautkramer KA, Kreznar JH, Romano KA, Vivas EI, Barrett-Wilt GA, Rabaglia ME, et al. Diet-Microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol Cell. 2016;64:982–92.
Vahamiko S, Laiho A, Lund R, Isolauri E, Salminen S, Laitinen K. The impact of probiotic supplementation during pregnancy on DNA methylation of obesity-related genes in mothers and their children. Eur J Nutr. 2018;58:367–77.
Acknowledgements
The research of A Tremblay is partly funded by the Canada Research Chair in Environment and Energy Balance. A. Marette’s research is funded by a CIHR/Pfizer research Chair in the pathogenesis of insulin resistance and cardiovascular diseases and by Sentinel North Program funded by the Canada First Research Excellence Fund. BSY Choi is funded by doctoral scholarships from Sentinel North and from CREATE-SMAART program funded by NSERC. L. Daoust is funded by the Jean-Paul-Houle fund from Université Laval.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, B.SY., Daoust, L., Pilon, G. et al. Potential therapeutic applications of the gut microbiome in obesity: from brain function to body detoxification. Int J Obes 44, 1818–1831 (2020). https://doi.org/10.1038/s41366-020-0618-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-0618-3
This article is cited by
-
The promise of the gut microbiome as part of individualized treatment strategies
Nature Reviews Gastroenterology & Hepatology (2022)