Abstract
Background
Obesity is a major factor behind insulin resistance. The validity of simple biochemical surrogate measures to estimate insulin resistance at the fat cell level is unclear.
Objective
To investigate if the surrogate measures HOMA-IR (glucose/insulin product) and Adipo-IR (fatty acids/insulin product) reflect insulin action on glucose/lipid metabolism in fat cells.
Design
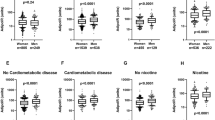
Insulin-induced lipogenesis and lipolysis inhibition (antilipolysis) in subcutaneous fat cells were investigated for sensitivity (reflecting receptor-near events) and responsiveness (i.e., maximum action reflecting distal post-receptor events) in 363 subjects. Results were compared with log10 transformed values for HOMA-IR and Adipo-IR.
Results
Individually, the four measures of in vitro insulin action on fat cells correlated significantly (p < 0.0001) but weakly with each other (adjusted r2 0.05–0.23). HOMA-IR and Adipo-IR correlated strongly with each other (adjusted r2 = 0.81). Using Spearman or simple linear regression all in vitro measures except antilipolytic responsiveness expressed per lipid weight, correlated significantly with Adipo-IR or HOMA-IR (p values <0.0001). Similar relationships remained after combined correction for age, body mass index and sex. Together, the four in vitro measures explained 50% of the variability in HOMA-IR and ADIPO-IR (p < 0.0001). Receiver-operating characteristic analysis showed good sensitivity and specificity for Adipo-IR and HOMA-IR to detect combined insulin resistance of antilipolysis and lipogenesis in fat cells (area under the curve = 0.8).
Conclusions
Insulin action at the receptor and post-receptor levels on lipolysis and lipogenesis in fat cells correlates significantly with Adipo-IR and HOMA-IR. Both surrogate measures give similar information about insulin resistance of glucose and lipid metabolism in fat cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Singh B, Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes. 2010;1:36–47.
Pacini G, Mari A. Methods for clinical assessment of insulin sensitivity and beta-cell function. Best Pract Res Clin Endocrinol Metab. 2003;17:305–22.
Smith U, Kahn BB. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J Intern Med. 2016;280:465–75.
Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends Endocrinol Metab. 2003;14:137–45.
Sondergaard E, Jensen MD. Quantification of adipose tissue insulin sensitivity. J Investig Med. 2016;64:989–91.
Ryden M, Petrus P, Andersson DP, Medina-Gomez G, Escasany E, Corrales Cordon P, et al. Insulin action is severely impaired in adipocytes of apparently healthy overweight and obese subjects. J Intern Med. 2019;285:578–88.
Hershkop K, Besor O, Santoro N, Pierpont B, Caprio S, Weiss R. Adipose insulin resistance in obese adolescents across the spectrum of glucose tolerance. J Clin Endocrinol Metab. 2016;101:2423–31.
Gastaldelli A, Gaggini M, DeFronzo RA. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results from the san antonio metabolism study. Diabetes. 2017;66:815–22.
Gastaldelli A, Gaggini M, Daniele G, Ciociaro D, Cersosimo E, Tripathy D, et al. Exenatide improves both hepatic and adipose tissue insulin resistance: a dynamic positron emission tomography study. Hepatology. 2016;64:2028–37.
Ter Horst KW, van Galen KA, Gilijamse PW, Hartstra AV, de Groot PF, van der Valk FM, et al. Methods for quantifying adipose tissue insulin resistance in overweight/obese humans. Int J Obes. 2017;41:1288–94.
Sondergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD. How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab. 2017;102:1193–9.
Ryden M, Arner P. Cardiovascular risk score is linked to subcutaneous adipocyte size and lipid metabolism. J Intern Med. 2017;282:220–8.
Ryden M, Arner P. Subcutaneous adipocyte lipolysis contributes to circulating lipid levels. Arterioscler Thromb Vasc Biol. 2017;37:1782–7.
Arner P, Ryden M. Fatty acids, obesity and insulin resistance. Obes Facts. 2015;8:147–55.
Hoffstedt J, Andersson DP, Eriksson Hogling D, Theorell J, Naslund E, Thorell A, et al. Long-term protective changes in adipose tissue after gastric bypass. Diabetes Care. 2017;40:77–84.
Arner P, Bolinder J, Hellmér J, Engfeldt P. Studies on human fat cell metabolism in small adipose tissue samples. In: Clarke. WL, Larner J, Pohl SL, Eds. Methods in Diabetes Research. Volume II: Clinical Methods. First Edition. New York, Chichester, Brisbane, Toronto, Singapore: Johh Wiley Sons; 1986. p. 233–58.
Arner P, Engfeldt P. Fasting-mediated alteration studies in insulin action on lipolysis and lipogenesis in obese women. Am J Physiol. 1987;253:E193–201.
Ryden M, Andersson DP, Bergstrom IB, Arner P. Adipose tissue and metabolic alterations: regional differences in fat cell size and number matter, but differently: a cross-sectional study. J Clin Endocrinol Metab. 2014;99:E1870–6.
Rizza RA, Mandarino LJ, Gerich JE. Mechanisms of insulin resistance in man. Assessment using the insulin dose-response curve in conjunction with insulin-receptor binding. Am J Med. 1981;70:169–76.
Kenakin T. Principles: receptor theory in pharmacology. Trends Pharmacol Sci. 2004;25:186–92.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Ferrannini E, Iozzo P, Virtanen KA, Honka MJ, Bucci M, Nuutila P. Adipose tissue and skeletal muscle insulin-mediated glucose uptake in insulin resistance: role of blood flow and diabetes. Am J Clin Nutr. 2018;108:749–58.
Virtanen KA, Lonnroth P, Parkkola R, Peltoniemi P, Asola M, Viljanen T, et al. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab. 2002;87:3902–10.
Brown RJ, Yanovski JA. Estimation of insulin sensitivity in children: methods, measures and controversies. Pediatr Diabetes. 2014;15:151–61.
Acknowledgements
The excellent technical assistance of Britt-Marie Leijonhufvud, Katarina Hertel, Yvonne Widlund, Eva Sjölin, Kerstin Wåhlén, Ana Maria Suzuki, Thais de Castro-Barbosa, and Gaby Åström is greatly appreciated. The study was supported by grants from Swedish Research Council, Knut and Alice Wallenberg’s foundation, Novo Nordisk Foundation (including the Tripartite Immuno-metabolism Consortium Grant Number NNF15CC0018486 and the MSAM consortium NNF15SA0018346), Stockholm County Council, CIMED, the Strategic Research Program in Diabetes at Karolinska Institutet and the Swedish Society of Medicine.
Author information
Authors and Affiliations
Contributions
PA and MR designed the study, wrote the first version of the MS and are the guarantors of the work. All authors analyzed data, contributed to further writing, and accepted the final version of the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rydén, M., Andersson, D.P. & Arner, P. Usefulness of surrogate markers to determine insulin action in fat cells. Int J Obes 44, 2436–2443 (2020). https://doi.org/10.1038/s41366-020-0592-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-0592-9
This article is cited by
-
Sex differences in adipose insulin resistance are linked to obesity, lipolysis and insulin receptor substrate 1
International Journal of Obesity (2024)
-
Correlation between serum uric acid and body fat distribution in patients with MAFLD
BMC Endocrine Disorders (2023)
-
Associations of objectively measured physical activity, sedentary time and cardiorespiratory fitness with adipose tissue insulin resistance and ectopic fat
International Journal of Obesity (2023)
-
A longitudinal study of the antilipolytic effect of insulin in women following bariatric surgery
International Journal of Obesity (2021)