Abstract
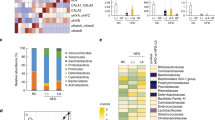
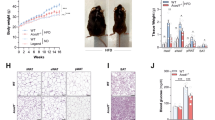
Lipocalin 2 (Lcn2), as an antimicrobial peptide is expressed in intestine, and the upregulation of intestinal Lcn2 has been linked to inflammatory bowel disease. However, the role of Lcn2 in shaping gut microbiota during diet-induced obesity (DIO) remains unknown. We found that short-term high fat diet (HFD) feeding strongly stimulates intestinal Lcn2 expression and secretion into the gut lumen. As the HFD feeding prolongs, fecal Lcn2 levels turn to decrease. Lcn2 deficiency accelerates the development of HFD-induced intestinal inflammation and microbiota dysbiosis. Moreover, Lcn2 deficiency leads to the remodeling of microbiota-derived metabolome, including decreased production of short-chain fatty acids (SCFAs) and SCFA-producing microbes. Most importantly, we have identified Lcn2-targeted bacteria and microbiota-derived metabolites that potentially play roles in DIO and metabolic dysregulation. Correlation analyses suggest that Lcn2-targeted Dubosiella and Angelakisella have a novel role in regulating SCFAs production and obesity. Our results provide a novel mechanism involving Lcn2 as an antimicrobial host factor in the control of gut microbiota symbiosis during DIO.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lin HV, Frassetto A, Kowalik EJ Jr, Nawrocki AR, Lu MM, Kosinski JR, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE. 2012;7:e35240.
Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829.
Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111:2247–52.
Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–81.
Garidou L, Pomie C, Klopp P, Waget A, Charpentier J, Aloulou M, et al. The Gut microbiota regulates intestinal CD4 T cells expressing RORgammat and controls metabolic disease. Cell Metab. 2015;22:100–12.
Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21:527–42.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31.
Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21.
Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2006;103:1834–9.
Zhang J, Wu Y, Zhang Y, Leroith D, Bernlohr DA, Chen X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol Endocrinol. 2008;22:1416–26.
Guo H, Jin D, Zhang Y, Wright W, Bazuine M, Brockman DA, et al. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes. 2010;59:1376–85.
Jin D, Guo H, Bu SY, Zhang Y, Hannaford J, Mashek DG, et al. Lipocalin 2 is a selective modulator of peroxisome proliferator-activated receptor-gamma activation and function in lipid homeostasis and energy expenditure. FASEB J. 2011;25:754–64.
Deis JA, Guo H, Wu Y, Liu C, Bernlohr DA, Chen X. Adipose Lipocalin 2 overexpression protects against age-related decline in thermogenic function of adipose tissue and metabolic deterioration. Mol Metab. 2019;24:18–29.
Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–305.
Xiao X, Yeoh BS, Vijay-Kumar M. Lipocalin 2: an emerging player in iron homeostasis and inflammation. Ann Rev Nutr. 2017;37:103–30.
Wilson BR, Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y. Siderophores in iron metabolism: from mechanism to therapy potential. Trends Mol Med. 2016;22:1077–90.
Stallhofer J, Friedrich M, Konrad-Zerna A, Wetzke M, Lohse P, Glas J, et al. Lipocalin-2 is a disease activity marker in inflammatory bowel disease regulated by IL-17A, IL-22, and TNF-alpha and modulated by IL23R genotype status. Inflamm Bowel Dis. 2015;21:2327–40.
Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS ONE. 2012;7:e44328.
Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476–86.
Moschen AR, Gerner RR, Wang J, Klepsch V, Adolph TE, Reider SJ, et al. Lipocalin 2 protects from inflammation and tumorigenesis associated with Gut microbiota alterations. Cell Host Microbe. 2016;19:455–69.
Lu Y, Yao D, Chen C. 2-hydrazinoquinoline as a derivatization agent for LC-MS-based metabolomic investigation of diabetic ketoacidosis. Metabolites. 2013;3:993–1010.
Sunil VR, Patel KJ, Nilsen-Hamilton M, Heck DE, Laskin JD, Laskin DL. Acute endotoxemia is associated with upregulation of lipocalin 24p3/Lcn2 in lung and liver. Exp Mol Pathol. 2007;83:177–87.
Glaros T, Fu Y, Xing J, Li L. Molecular mechanism underlying persistent induction of LCN2 by lipopolysaccharide in kidney fibroblasts. PLoS ONE. 2012;7:e34633.
Borkham-Kamphorst E, van de Leur E, Zimmermann HW, Karlmark KR, Tihaa L, Haas U, et al. Protective effects of lipocalin-2 (LCN2) in acute liver injury suggest a novel function in liver homeostasis. Biochimica Biophys Acta. 2013;1832:660–73.
Kang SS, Ren Y, Liu CC, Kurti A, Baker KE, Bu G, et al. Lipocalin-2 protects the brain during inflammatory conditions. Mol Psychiatry. 2018;23:344–50.
Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–24 e1-2.
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4.
Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–70.
Smith BJ, Miller RA, Ericsson AC, Harrison DE, Strong R, Schmidt TM. Changes in the gut microbiota and fermentation products associated with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019;19:130.
Kim K-A, Gu W, Lee I-A, Joh E-H, Kim D-H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE. 2012;7:e47713.
Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734.
Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, et al. Population-based metagenomics analysis reveals markers for gut microbiome composiiton and diversity. Science. 2016;352:565–9.
Louis P, Flint HJ. Formation of porpionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19:29–41.
Holman DB, Gzyl KE. A meta-analysis of the bovine gastrointestinal tract microbiota. FEMS Microbiol Ecol. 2019;95:fiz072.
Hillmer RA, Tsuda K, Rallapalli G, Asai S, Truman W, Papke MD, et al. The highly buffered Arabidopsis immune signaling network conceals the functions of its components. PLoS Genet. 2017;13:e1006639.
Cox LM, Sohn J, Tyrrell KL, Citron DM, Lawson PA, Patel NB, et al. Description of two novel members of the family Erysipelotrichaceae: Ileibacterium valens gen. nov., sp. nov. and Dubosiella newyorkensis, gen. nov., sp. nov., from the murine intestine, and emendation to the description of Faecalibaculum rodentium. Int J Syst Evol Microbiol. 2017;67:1247–54.
Mailhe M, Ricaboni D, Vitton V, Cadoret F, Fournier PE, Raoult D. ‘Angelakisella massiliensis’ gen. nov., sp. nov., a new bacterial species isolated from human ileum. New Microbes New Infect. 2017;16:51–3.
Shahi SK, Freedman SN, Mangalam AK. Gut microbiome in multiple sclerosis: the players involved and the roles they play. Gut Microbes. 2017;8:607–15.
Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–5.
Everard A, Lazarevic V, Gaia N, Johansson M, Stahlman M, Backhed F, et al. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014;8:2116–30.
Mao Z, Ren Y, Zhang Q, Dong S, Han K, Feng G, et al. Glycated fish protein supplementation modulated gut microbiota composition and reduced inflammation but increased accumulation of advanced glycation end products in high-fat diet fed rats. Food Funct. 2019;10:3439–51.
Acknowledgements
This work was supported by Allen Foundation Grant awarded to XC, the General Mills Foundation Chair in Genomics for Healthful Foods to XC, NIDDK Grant (R01 DK123042) awarded to XC, and the Doctoral Dissertation Fellowship from the University of Minnesota to XQ.
Author information
Authors and Affiliations
Contributions
XQ designed and performed the experiments, analyzed the data including 16S rRNA sequencing data, and wrote this article. MM analyzed 16S rRNA sequencing data and wrote part of the Methods. XL analyzed 16S rRNA sequencing data. XQ and YL performed statistical analysis. YM and HG performed the experiments. MS performed IHC data analysis and interpretation. DBA conceived and designed the experiments. CC conceived and designed the experiments. SS conceived and designed 16S rRNA sequencing data analysis. XC conceived and designed the experiments, analyzed the data, and wrote the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41366_2020_712_MOESM1_ESM.pdf
Identification of gut microbiota and microbial metabolites regulated by an antimicrobial peptide lipocalin 2 in high fat diet-induced obesity
Rights and permissions
About this article
Cite this article
Qiu, X., Macchietto, M.G., Liu, X. et al. Identification of gut microbiota and microbial metabolites regulated by an antimicrobial peptide lipocalin 2 in high fat diet-induced obesity. Int J Obes 45, 143–154 (2021). https://doi.org/10.1038/s41366-020-00712-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-00712-2
This article is cited by
-
Gut microbiota and neonatal acute kidney injury biomarkers
Pediatric Nephrology (2023)
-
Endurance exercise ameliorates Western diet–induced atherosclerosis through modulation of microbiota and its metabolites
Scientific Reports (2022)
-
Pomegranate peel anthocyanins prevent diet-induced obesity and insulin resistance in association with modulation of the gut microbiota in mice
European Journal of Nutrition (2022)