Abstract
Objectives
We hypothesised that maternal diet-induced-obesity has adverse consequences for offspring energy expenditure and susceptibility to obesity in adulthood, and that the prebiotic polydextrose (PDX) would prevent the consequences of programming by maternal obesity.
Methods
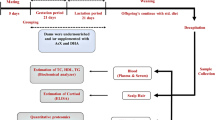
Female mice were fed a control (Con) or obesogenic diet (Ob) for 6 weeks prior to mating and throughout pregnancy and lactation. Half the obese dams were supplemented with 5% PDX (ObPDX) in drinking water throughout pregnancy and lactation. Offspring were weaned onto standard chow. At 3 and 6 months, offspring energy intake (EI) and energy expenditure (EE by indirect calorimetry) were measured, and a glucose-tolerance test performed. Offspring of control (OffCon), obese (OffOb) and PDX supplemented (OffObP) dams were subsequently challenged for 3 weeks with Ob, and energy balanced reassessed. Potential modifiers of offspring energy balance including gut microbiota and biomarkers of mitochondrial activity were also evaluated.
Results
Six-month-old male OffOb demonstrated increased bodyweight (BW, P < 0.001) and white adipose tissue mass (P < 0.05), decreased brown adipose tissue mass (BAT, P < 0.01), lower night-time EE (P < 0.001) versus OffCon, which were prevented in OffObP. Both male and female OffOb showed abnormal glucose-tolerance test (peak [Glucose] P < 0.001; AUC, P < 0.05) which was prevented by PDX. The Ob challenge resulted in greater BW gain in both male and female OffOb versus OffCon (P < 0.05), also associated with increased EI (P < 0.05) and reduced EE in females (P < 0.01). OffObP were protected from accelerated BW gain on the OB diet compared with controls, associated with increased night-time EE in both male (P < 0.05) and female OffObP (P < 0.001). PDX also prevented an increase in skeletal muscle mtDNA copy number in OffOb versus OffCon (P < 0.01) and increased the percentage of Bacteroides cells in faecal samples from male OffObP relative to controls.
Conclusions
Maternal obesity adversely influences adult offspring energy balance and propensity for obesity, which is ameliorated by maternal PDX treatment with associated changes in gut microbiota composition and skeletal muscle mitochondrial function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Poston L, Caleyachetty R, Cnattingius S, Corvalan C, Uauy R, Herring S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016;4:1025–36.
Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5:53–64.
Heslehurst N, Vieira R, Akhter Z, Bailey H, Slack E, Ngongalah L, et al. The association between maternal body mass index and child obesity: a systematic review and meta-analysis. PLoS Med. 2019;16:e1002817.
Scholtens DM, Kuang A, Lowe LP, Hamilton J, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal glycemia and childhood glucose metabolism. Diabetes Care. 2019;42:381–92.
Dalrymple K, Thompson J, Begum S, Godfrey KM, Poston L, Seed, PT. et al. Relationships of maternal body mass index and plasma biomarkers with childhood body mass index and adiposity at 6 years; the Children of SCOPE study. Int J Pead Ob. 2019;14:e12537.
Hanson M, Barker M, Dodd JM, Kumanyika S, Norris S, Steegers E, et al. Interventions to prevent maternal obesity before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol. 2017;5:65–76.
do Carmo MM, Walker JC, Novello D, Caselato VM, Sgarbieri VC, Ouwehand AC, et al. Polydextrose: physiological function, and effects on health. Nutrients. 2016;8. https://doi.org/10.3390/nu809055300.
Ibarra A, Astbury NM, Olli K, Alhoniemi E, Tiihonen K. Effects of polydextrose on different levels of energy intake. A systematic review and meta-analysis. Appetite. 2015;87:30–7.
Ibarra A, Astbury NM, Olli K, Alhoniemi E, Tiihonen K. Effect of polydextrose on subjective feelings of appetite during the satiation and satiety periods: a systematic review and meta-analysis. Nutrients. 2016;8. https://doi.org/10.3390/nu8010045.
Ibarra A, Olli K, Pasman W, Hendriks H, Alhoniemi E, Raza GS, et al. Effects of polydextrose with breakfast or with a midmorning preload on food intake and other appetite-related parameters in healthy normal-weight and overweight females: An acute, randomized, double-blind, placebo-controlled, and crossover study. Appetite. 2017;110:15–24.
Olli K, Salli K, Alhoniemi E, Saarinen M, Ibarra A, Vasankari T, et al. Postprandial effects of polydextrose on satiety hormone responses and subjective feelings of appetite in obese participants. Nutr J. 2015;14:2.
Airaksinen K, Jokkala J, Ahonen I, Auriola S, Kolehmainen M, Hanhineva K. et al. High-fat diet, betaine, and polydextrose induce changes in adipose tissue inflammation and metabolism in C57BL/6J mice. Mol Nutr Food Res. 2018;62:e1800455. https://doi.org/10.1002/mnfr.201800455.
Raza GS, Putaala H, Hibberd AA, Alhoniemi E, Tiihonen K, Makela KA, et al. Polydextrose changes the gut microbiome and attenuates fasting triglyceride and cholesterol levels in Western diet fed mice. Sci Rep. 2017;7:5294.
Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–92.
Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C, et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52:913–20.
Oben JA, Patel T, Mouralidarane A, Samuelsson AM, Matthews P, Pombo J, et al. Maternal obesity programmes offspring development of non-alcoholic fatty pancreas disease. Biochem Biophys Res Commun. 2010;394:24–8.
Mouralidarane A, Soeda J, Visconti-Pugmire C, Samuelsson AM, Pombo J, Maragkoudaki X, et al. Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology. 2013;58:128–38.
Lagisz M, Blair H, Kenyon P, Uller T, Raubenheimer D, Nakagawa S. Little appetite for obesity: meta-analysis of the effects of maternal obesogenic diets on offspring food intake and body mass in rodents. Int J Obes. 2015;39:1669–78.
Witaicenis A, Fruet AC, Salem L, Di, Stasi LC. Dietary polydextrose prevents inflammatory bowel disease in trinitrobenzenesulfonic acid model of rat colitis. J Med Food. 2010;13:1391–6.
Stenman LK, Waget A, Garret C, Briand F, Burcelin R, Sulpice T, et al. Probiotic B420 and prebiotic polydextrose improve efficacy of antidiabetic drugs in mice. Diabetol Metab Syndr. 2015;7:75.
Malik AN, Czajka A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion. 2013;13:481–92.
Czajka A, Malik AN. Hyperglycemia induced damage to mitochondrial respiration in renal mesangial and tubular cells: Implications for diabetic nephropathy. Redox Biol. 2016;10:100–7.
Rigottier-Gois L, Bourhis AG, Gramet G, Rochet V, Dore J. Fluorescent hybridisation combined with flow cytometry and hybridisation of total RNA to analyse the composition of microbial communities in human faeces using 16S rRNA probes. FEMS Microbiol Ecol. 2003;43:237–45.
Shimomura Y, Maeda K, Nagasaki M, Matsuo Y, Murakami T, Bajotto G, et al. Attenuated response of the serum triglyceride concentration to ingestion of a chocolate containing polydextrose and lactitol in place of sugar. Biosci Biotechnol Biochem. 2005;69:1819–23.
Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1.
Roman AS, Rebarber A, Fox NS, Klauser CK, Istwan N, Rhea D, et al. The effect of maternal obesity on pregnancy outcomes in women with gestational diabetes. J Matern Fetal Neonatal Med. 2011;24:723–7.
Bo S, Menato G, Signorile A, Bardelli C, Lezo A, Gallo ML, et al. Obesity or diabetes: what is worse for the mother and for the baby? Diabetes Metab. 2003;29:175–8.
Al-Sobayil K, Zeitoun M, Abdel-Salam A. Effectiveness of a functional synbiotic syrup on pregnancy rate, neonatal birth weight and progesterone profile of oestrous-synchronized Najdi ewes. J Food Agric Environ. 2010;8:80–5.
Castillo-Laura H, Santos IS, Quadros LC, Matijasevich A. Maternal obesity and offspring body composition by indirect methods: a systematic review and meta-analysis. Cad Saude Publica. 2015;31:2073–92.
Taylor PD, McConnell J, Khan IY, Holemans K, Lawrence KM, Asare-Anane H, et al. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288:R134–9.
Rising R, Lifshitz F. Lower energy expenditures in infants from obese biological mothers. Nutr J. 2008;7:15.
Lau SM, Lin S, Stokes RA, Cheng K, Baldock PA, Enriquez RF, et al. Synergistic effects of genetic beta cell dysfunction and maternal glucose intolerance on offspring metabolic phenotype in mice. Diabetologia. 2011;54:910–21.
Borengasser SJ, Lau F, Kang P, Blackburn ML, Ronis MJ, Badger TM, et al. Maternal obesity during gestation impairs fatty acid oxidation and mitochondrial SIRT3 expression in rat offspring at weaning. PLoS ONE. 2011;6:e24068.
Ong ZY, Muhlhausler BS. Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J. 2011;25:2167–79.
Gugusheff JR, Bae SE, Rao A, Clarke IJ, Poston L, Taylor PD, et al. Sex and age-dependent effects of a maternal junk food diet on the mu-opioid receptor in rat offspring. Behav Brain Res. 2016;301:124–31.
Bayol SA, Farrington SJ, Stickland NC. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr. 2007;98:843–51.
Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, Poston L, et al. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS ONE. 2009;4:e5870.
Bogardus C, Lillioja S, Mott D, Zawadzki J, Young A, Abbott W. Evidence for reduced thermic effect of insulin and glucose infusions in Pima Indians. J Clin Invest. 1985;75:1264–9.
Schutz Y, Bessard T, Jequier E. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am J Clin Nutr. 1984;40:542–52.
Golay A, Schutz Y, Felber JP, Jallut D, Jequier E. Blunted glucose-induced thermogenesis in ‘overweight’ patients: a factor contributing to relapse of obesity. Int J Obes. 1989;13:767–75.
Fernandes C, Grayton H, Poston L, Samuelsson AM, Taylor PD, Collier DA, et al. Prenatal exposure to maternal obesity leads to hyperactivity in offspring. Mol Psychiatry. 2012;17:1159–60.
Rooney JP, Ryde IT, Sanders LH, Howlett EH, Colton MD, Germ KE, et al. PCR based determination of mitochondrial DNA copy number in multiple species. Methods Mol Biol. 2015;1241:23–38.
Al-Kafaji G, Sabry MA, Bakhiet M. Increased expression of mitochondrial DNA-encoded genes in human renal mesangial cells in response to high glucose-induced reactive oxygen species. Mol Med Rep. 2016;13:1774–80.
Masser DR, Clark NW, Van Remmen H, Freeman WM. Loss of the antioxidant enzyme CuZnSOD (Sod1) mimics an age-related increase in absolute mitochondrial DNA copy number in the skeletal muscle. Age. 2016;38:323–33.
Malik AN, Parsade CK, Ajaz S, Crosby-Nwaobi R, Gnudi L, Czajka A, et al. Altered circulating mitochondrial DNA and increased inflammation in patients with diabetic retinopathy. Diabetes Res Clin Pract. 2015;110:257–65.
Picca A, Lezza AMS, Leeuwenburgh C, Pesce V, Calvani R, Bossola M, et al. Circulating mitochondrial DNA at the crossroads of mitochondrial dysfunction and inflammation during aging and muscle wasting disorders. Rejuvenation Res. 2018;21:350–9.
de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108:1379–85.
Samuelsson AM, Clark J, Rudyk O, Shattock MJ, Bae SE, South T, et al. Experimental hyperleptinemia in neonatal rats leads to selective leptin responsiveness, hypertension, and altered myocardial function. Hypertension. 2013;62:627–33.
Samuelsson AM, Morris A, Igosheva N, Kirk SL, Pombo JM, Coen CW, et al. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension. 2010;55:76–82.
Mohle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, et al. Ly6C(hi) monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 2016;15:1945–56.
Rodriguez VM, Portillo MP, Pico C, Macarulla MT, Palou A. Olive oil feeding up-regulates uncoupling protein genes in rat brown adipose tissue and skeletal muscle. Am J Clin Nutr. 2002;75:213–20.
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–40.
Hoeks J, Arany Z, Phielix E, Moonen-Kornips E, Hesselink MK, Schrauwen P. Enhanced lipid-but not carbohydrate-supported mitochondrial respiration in skeletal muscle of PGC-1alpha overexpressing mice. J Cell Physiol. 2012;227:1026–33.
Zhu MJ, Du M, Ford SP. Impacts of maternal obesity on placental and gut inflammation and health. J Anim Sci. 2013;92:1840–9. https://doi.org/10.2527/jas.2013-7106.
Myles IA, Fontecilla NM, Janelsins BM, Vithayathil PJ, Segre JA, Datta SK. Parental dietary fat intake alters offspring microbiome and immunity. J Immunol. 2013;191:3200–9.
Stolarczyk E, Vong CT, Perucha E, Jackson I, Cawthorne MA, Wargent ET, et al. Improved insulin sensitivity despite increased visceral adiposity in mice deficient for the immune cell transcription factor T-bet. Cell Metab. 2013;17:520–33.
Soderborg TK, Clark SE, Mulligan CE, Janssen RC, Babcock L, Ir D, et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun. 2018;9:4462.
Laitinen K, Poussa T, Isolauri E, Nutrition AMI, Intestinal Microbiota G. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br J Nutr. 2009;101:1679–87.
Collado MC, Cernada M, Bauerl C, Vento M, Perez-Martinez G. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes. 2012;3:352–65.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3.
Acknowledgements
This work was supported by a BBSRC industrial CASE studentship (BB/G017093/1) with Tate & Lyle PLC; Tommy’s Charity; European Union 7th Framework Programme (FP7/2007–2013) project EarlyNutrition under grant agreement No. 289346, Medical Research Council UK; Newton Fund (Partnership Project grant) RCUK-CONACYT Research Partnerships MR/N029259/1. Polydextrose was provided by Tate & Lyle. This study also received support from the NIHR Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed in this publication are those of the author and not necessarily those of the NHS, the NIHR, or the Department of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Maragkoudaki, X., Naylor, M., Papacleovoulou, G. et al. Supplementation with a prebiotic (polydextrose) in obese mouse pregnancy improves maternal glucose homeostasis and protects against offspring obesity. Int J Obes 44, 2382–2393 (2020). https://doi.org/10.1038/s41366-020-00682-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-00682-5