Abstract
Background/objectives
Mitochondrial dysfunction, oxidative stress, or fatty liver are the key pathophysiological features for insulin resistance and obesity. Dehydroepiandrosterone (DHEA) can ameliorate obesity and insulin resistance; however, the mechanisms of these actions are poorly understood. The present study aimed to investigate the effect and possible mechanism of DHEA against glycolipid metabolic disorder and insulin resistance.
Subjects/methods
Rats fed a high-fat diet (HFD) and palmitic acid (PA)-induced BRL-3A cells were employed to analyze the effect of DHEA on factors related to metabolic disorder and insulin resistance in vivo and in vitro.
Results
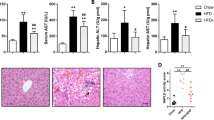
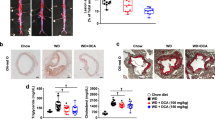
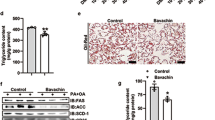
DHEA prevented lipid metabolism disorders by enhancing phospho (p)-protein kinase AMP-activated catalytic subunit alpha (AMPKα) (Thr172) protein level and its downstream lipid metabolism-related factors in liver of rats fed an HFD or in PA-induced BRL-3A cells. Meanwhile, DHEA ameliorated mitochondrial dysfunction through activation of the AMPK-peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α)-nuclear respiratory factor-1 (NRF-1) pathway, which represented as the enhancing of the mtDNA copy number, ATP level, and membrane potential, and decreasing of reactive oxygen species production. Moreover, DHEA alleviated insulin resistance via increasing the phosphorylated insulin receptor substrate 1 (p-IRS1) (Tyr612) level and decreasing that of p-IRS1 (Ser307) level in liver of rats fed an HFD or in PA-induced BRL-3A cells, which subsequently enhanced p-protein kinase B (AKT) (Ser473) and membrane glucose transporter type 2 (GLUT2) expression levels.
Conclusions
The protective effect of DHEA on high-fat-induced hepatic glycolipid metabolic disorder and insulin resistance are achieved through activation of the AMPK-PGC-1α-NRF-1 and IRS1-AKT-GLUT2 signaling pathways. The results provide compelling evidence for the mechanism by which DHEA prevents glycolipid metabolic disorder, and suggest its potential applications for controlling diabetes and obesity in animals and humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Stein CJ, Colditz GA. The epidemic of obesity. J Clin Endocrinol Metab. 2000;172:78–9.
Hernándezaguilera A, Rull A, Rodríguezgallego E, Rieraborrull M, Lucianomateo F, Camps J, et al. Mitochondrial dysfunction: a basic mechanism in inflammation-related non-communicable diseases and therapeutic opportunities. Mediators Inflamm. 2013;2013:135698.
Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, et al. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem. 2009;284:14809–18.
Mollica MP, Mattace Raso G, Cavaliere G, Trinchese G, De Filippo C, Aceto S, et al. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes. 2017;66:1405–18.
De Marchi U, Castelbou C, Demaurex N. Uncoupling protein 3 (UCP3) modulates the activity of Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) by decreasing mitochondrial ATP production. J Biol Chem. 2011;286:32533–41.
Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–82.
Labrie F, Belanger A, Luu-The V, Labrie C, Simard J, Cusan L, et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–8.
Bacsi K, Kosa J, Lazary A, Horvath H, Balla B, Lakatos P, et al. Significance of dehydroepiandrosterone and dehydroepiandrosterone sulfate in different diseases. Orv Hetil. 2007;148:651–7.
Coleman DL, Schwizer RW, Leiter EH. Effect of genetic background on the therapeutic effects of dehydroepiandrosterone (DHEA) in diabetes-obesity mutants and in aged normal mice. Diabetes. 1984;33:26–32.
Jarrar D, Wang P, Cioffi WG, Bland KI, Chaudry IH. Mechanisms of the salutary effects of dehydroepiandrosterone after trauma-hemorrhage: direct or indirect effects on cardiac and hepatocellular functions. Arch Surg. 2000;135:416–22.
Buffington CK, Pourmotabbed G, Kitabchi AE. Case report: amelioration of insulin resistance in diabetes with dehydroepiandrosterone. Am J Med Sci. 1993;306:320–4.
Li L, Ge C, Wang D, Yu L, Zhao J, Ma H. Dehydroepiandrosterone reduces accumulation of lipid droplets in primary chicken hepatocytes by biotransformation mediated via the cAMP/PKA-ERK1/2 signaling pathway. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:625–38.
Li L, Zhang H, Yao Y, Yang Z, Ma H. (−)−Hydroxycitric acid suppresses lipid droplet accumulation and accelerates energy metabolism via activation of the adiponectin-AMPK signaling pathway in broiler chickens. J Agric Food Chem. 2019;67:3188–97.
Ding X, Wang D, Li L, Ma H. Dehydroepiandrosterone ameliorates H2O2-induced Leydig cells oxidation damage and apoptosis through inhibition of ROS production and activation of PI3K/Akt pathways. Int J Biochem Cell Biol. 2016;70:126–39.
Campbell CS, Caperuto LC, Hirata AE, Araujo EP, Velloso LA, Saad MJ, et al. The phosphatidylinositol/AKT/atypical PKC pathway is involved in the improved insulin sensitivity by DHEA in muscle and liver of rats in vivo. Life Sci. 2004;76:57–70.
Formoso G, Chen H, Kim JA, Montagnani M, Consoli A, Quon MJ. Dehydroepiandrosterone mimics acute actions of insulin to stimulate production of both nitric oxide and endothelin 1 via distinct phosphatidylinositol 3-kinase- and mitogen-activated protein kinase-dependent pathways in vascular endothelium. Mol Endocrinol. 2006;20:1153–63.
Perrini S, Natalicchio A, Laviola L, Belsanti G, Montrone C, Cignarelli A, et al. Dehydroepiandrosterone stimulates glucose uptake in human and murine adipocytes by inducing GLUT1 and GLUT4 translocation to the plasma membrane. Diabetes. 2004;53:41–52.
Hakkak R, Bell A, Korourian S. Dehydroepiandrosterone (DHEA) feeding protects liver steatosis in obese breast cancer rat model. Sci Pharm. 2017;85:13.
Rathinam A, Pari L. Myrtenal ameliorates hyperglycemia by enhancing GLUT2 through Akt in the skeletal muscle and liver of diabetic rats. Chem Biol Interact. 2016;256:161–6.
Kiersztan A, Trojan N, Tempes A, Nalepa P, Sitek J, Winiarska K, et al. DHEA supplementation to dexamethasone-treated rabbits alleviates oxidative stress in kidney-cortex and attenuates albuminuria. J Steroid Biochem Mol Biol. 2017;174:17–26.
Kang J, Ge C, Yu L, Li L, Ma H. Long-term administration of dehydroepiandrosterone accelerates glucose catabolism via activation of PI3K/Akt-PFK-2 signaling pathway in rats fed a high-fat diet. PLoS ONE. 2016;11:e0159077.
Jorgensen SB, Richter EA, Wojtaszewski JF. Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J Physiol. 2006;574:17–31.
Madiraju AK, Alves T, Zhao X, Cline GW, Zhang D, Bhanot S, et al. Argininosuccinate synthetase regulates hepatic AMPK linking protein catabolism and ureagenesis to hepatic lipid metabolism. Proc Natl Acad Sci USA. 2016;113:E3423–30.
Foretz M, Even PC, Viollet B. AMPK activation reduces hepatic lipid content by increasing fat oxidation in vivo. Int J Mol Sci. 2018;19:2826.
Fu M, Zhang W, Wu L, Yang G, Li H, Wang R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci USA. 2012;109:2943–8.
Fiedorczuk K, Letts JA, Degliesposti G, Kaszuba K, Skehel M, Sazanov LA. Atomic structure of the entire mammalian mitochondrial complex I. Nature. 2016;538:406–10.
Lim W, Ryu S, Bazer FW, Kim SM, Song G. Chrysin attenuates progression of ovarian cancer cells by regulating signaling cascades and mitochondrial dysfunction. J Cell Physiol. 2018;233:3129–40.
Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, et al. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55:120–7.
Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, et al. PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal. 2010;13:1011–22.
Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–60.
Kang D, Kim SH, Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion. 2007;7:39–44.
Garcia-Roves PM, Osler ME, Holmstrom MH, Zierath JR. Gain-of-function R225Q mutation in AMP-activated protein kinase gamma3 subunit increases mitochondrial biogenesis in glycolytic skeletal muscle. J Biol Chem. 2008;283:35724–34.
Herzig RP, Scacco S, Scarpulla RC. Sequential serum-dependent activation of CREB and NRF-1 leads to enhanced mitochondrial respiration through the induction of cytochrome c. J Biol Chem. 2000;275:13134–41.
Kaarsholm NC, Lin S, Yan L, Kelly T, van Heek M, Mu J, et al. Engineering glucose responsiveness into insulin. Diabetes. 2018;67:299–308.
Raubenheimer PJ, Nyirenda MJ, Walker BR. A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes. 2006;55:2015–20.
Coll T, Eyre E, Rodriguez-Calvo R, Palomer X, Sanchez RM, Merlos M, et al. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem. 2008;283:11107–16.
Qi G, Guo R, Tian H, Li L, Liu H, Mi Y, et al. Nobiletin protects against insulin resistance and disorders of lipid metabolism by reprogramming of circadian clock in hepatocytes. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:549–62.
Cheng KK, Iglesias MA, Lam KS, Wang Y, Sweeney G, Zhu W, et al. APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 2009;9:417–27.
Chen K, Li G, Geng F, Zhang Z, Li J, Yang M, et al. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K-Akt signaling in diabetic rats. Apoptosis. 2014;19:946–57.
Kim KY, Baek A, Hwang JE, Choi YA, Jeong J, Lee MS, et al. Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res. 2009;69:4018–26.
Utriainen T, Takala T, Luotolahti M, Rönnemaa T, Laine H, Ruotsalainen U, et al. Insulin resistance characterizes glucose uptake in skeletal muscle but not in the heart in NIDDM. Diabetologia. 1998;41:555–9.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number 31572483); the Fundamental Research Funds for the Central Universities (grant number KYDZ201901); the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD); and the Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX18_0715).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, L., Yao, Y., Zhao, J. et al. Dehydroepiandrosterone protects against hepatic glycolipid metabolic disorder and insulin resistance induced by high fat via activation of AMPK-PGC-1α-NRF-1 and IRS1-AKT-GLUT2 signaling pathways. Int J Obes 44, 1075–1086 (2020). https://doi.org/10.1038/s41366-019-0508-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0508-8