Abstract
Objectives
A high dairy protein intake in infancy, maternal pre-pregnancy BMI, and delivery mode are documented early programming factors that modulate the later risk of obesity and other health outcomes, but the mechanisms of action are not understood.
Methods
The Childhood Obesity Project is a European multicenter, double-blind, randomized clinical trial that enrolled healthy infants. Participating infants were either breastfed (BF) or randomized to receive higher (HP) or lower protein (LP) content formula in the first year of life. At the ages 5.5 years (n = 276) and 8 years (n = 232), we determined plasma metabolites by liquid chromatography tandem-mass-spectrometry of which 226 and 185 passed quality control at 5.5 years and 8 years, respectively. We assessed the effects of infant feeding, maternal pre-pregnancy BMI, smoking in pregnancy, delivery mode, parity, birth weight and length, and weight gain (0–24 months) on the metabolome at 5.5 and 8 years.
Results
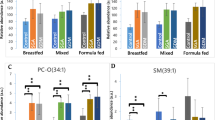
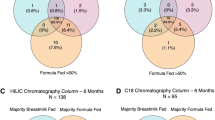
At 5.5 years, plasma alpha-ketoglutarate and the acylcarnitine/BCAA ratios tended to be higher in the HP than in the LP group, but no metabolite reached statistical significance (Pbonferroni>0.09). There were no group differences at 8 years. Quantification of the impact of early programming factors revealed that the intervention group explained 0.6% of metabolome variance at both time points. Except for country of residence that explained 16% and 12% at 5.5 years and 8 years, respectively, none of the other factors explained considerably more variance than expected by chance.
Conclusions
Plasma metabolome was largely unaffected by feeding choice and other early programming factors and we could not prove the existence of a long term programming effect of the plasma metabolome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Seidell JC, Halberstadt J. Obesity: the obesity epidemic in the USA-no end in sight?. Nat Rev Endocrinol. 2016;12:499–500.
Dörner G. Perinatal hormone levels and brain organization. In: Anatomical neuroendocrinology. Karger, Basel: Int. Conf. Neurobiology of CNS-Hormone InteractionsG Dörner; 1975, pp. 245–52.
Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601.
Lucas A. Programming by early nutrition in man. Ciba Found Symp. 1991;156:38–50. discussion 50-5
Hellmuth C, Uhl O, Kirchberg FF, Grote V, Weber M, Rzehak P, et al. Effects of early nutrition on the infant metabolome. Nestle Nutrition Institute Workshop Series. 2016;85:89–100.
Koletzko B, Brands B, Grote V, Kirchberg FF, Prell C, Rzehak P, et al. Long-term health impact of early nutrition: the power of programming. Ann Nutr Metabolism. 2017;70:161–9.
Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, et al. Can infant feeding choices modulate later obesity risk? Am J Clin Nutr. 2009;89:1502S–8S.
Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, et al. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr. 2009;89:1836–45.
Weber M, Grote V, Closa-Monasterolo R, Escribano J, Langhendries JP, Dain E, et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr. 2014;99:1041–51.
Fiehn O. Metabolomics–the link between genotypes and phenotypes. Plant Mol Biol. 2002;48:155–71.
Nicholson JK. Global systems biology, personalized medicine and molecular epidemiology. Mol Syst Biol. 2006;2:1–6. https://www.embopress.org/doi/pdf/10.1038/msb4100095.
Ritchie MD, Holzinger ER, Li R, Pendergrass SA, Kim D. Methods of integrating data to uncover genotype-phenotype interactions. Nat Rev Genet. 2015;16:85–97.
de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organization. 2007;85:660–7.
de Onis M, Onyango AW, Van den Broeck J, Chumlea WC, Martorell R. Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr Bull. 2004;25:S27–36.
Fages A, Ferrari P, Monni S, Dossus L, Floegel A, Mode N, et al. Investigating sources of variability in metabolomic data in the EPIC study: the Principal Component Partial R-square (PC-PR2) method. Metabolomics. 2014;10:1074–83.
Kirchberg FF, Harder U, Weber M, Grote V, Demmelmair H, Peissner W, et al. Dietary protein intake affects amino acid and acylcarnitine metabolism in infants aged 6 months. J Clin Endocrinol Metabolism. 2015;100:149–58.
Kerbey AL, Randle PJ, Cooper RH, Whitehouse S, Pask HT, Denton RM. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochemical J. 1976;154:327–48.
Yao K, Yin Y, Li X, Xi P, Wang J, Lei J, et al. Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino Acids. 2012;42:2491–500.
Hammarqvist F, Wernerman J, von der Decken A, Vinnars E. Alpha-ketoglutarate preserves protein synthesis and free glutamine in skeletal muscle after surgery. Surgery. 1991;109:28–36.
Cai X, Zhu C, Xu Y, Jing Y, Yuan Y, Wang L, et al. Alpha-ketoglutarate promotes skeletal muscle hypertrophy and protein synthesis through Akt/mTOR signaling pathways. Sci Rep. 2016;6:26802.
Hosten AO. BUN and Creatinine. In: Walker HK, Hall WD, Hurst JW (eds). Clinical methods: the history, physical, and laboratory examinations. 3rd edn. Boston: Butterworths; 1990. https://www.ncbi.nlm.nih.gov/books/NBK305/.
Jonas A. Lecithin cholesterol acyltransferase. Biochim et Biophys Acta. 2000;1529:245–56.
Park CS. Influence of dietary protein on blood cholesterol and related metabolites of growing calves. J Animal Sci. 1985;61:924–30.
Yashiro M, Kimura S. Effect of voluntary exercise and dietary protein levels on serum lipoprotein distributions and lecithin: cholesterol acyltransferase (LCAT) activity of mice. J Nutr Sci Vitaminol. 1980;26:59–69.
Lamri MY, Meghelli-Bouchenak M, Boualga A, Belleville J, Prost J. Time course of changes in rat serum lecithin-cholesterol acyl-transferase activity and high-density-lipoprotein composition during the consumption of two different low-protein diets followed by a balanced diet. Nutrition. 1995;11:444–9.
Flacking R, Nyqvist KH, Ewald U. Effects of socioeconomic status on breastfeeding duration in mothers of preterm and term infants. Eur J Public Health. 2007;17:579–84.
Flacking R, Dykes F, Ewald U. The influence of fathers' socioeconomic status and paternity leave on breastfeeding duration: a population-based cohort study. Scand J Public Health. 2010;38:337–43.
Ang JE, Revell V, Mann A, Mantele S, Otway DT, Johnston JD, et al. Identification of human plasma metabolites exhibiting time-of-day variation using an untargeted liquid chromatography-mass spectrometry metabolomic approach. Chronobiol Int. 2012;29:868–81.
Bundy JG, Davey MP, Viant MR. Environmental metabolomics: a critical review and future perspectives. Metabolomics. 2008;5:3.
Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–9.
Vernocchi P, Del Chierico F, Putignani L. Gut microbiota profiling: metabolomics based approach to unravel compounds affecting human health. Front Microbiol. 2016;7:1144.
Efsa Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for protein. EFSA J. 2012;10:2557.
Adeva-Andany MM, Lopez-Maside L, Donapetry-Garcia C, Fernandez-Fernandez C, Sixto-Leal C. Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids. 2017;49:1005–28.
Zhao Y, Denne SC, Harris RA. Developmental pattern of branched-chain 2-oxo acid dehydrogenase complex in rat liver and heart. Biochem J. 1993;290:395–9.
Garcia-Perez I, Posma JM, Gibson R, Chambers ES, Hansen TH, Vestergaard H, et al. Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endocrinol. 2017;5:184–95.
Bhupathiraju SN, Hu FB. One (small) step towards precision nutrition by use of metabolomics. Lancet Diabetes Endocrinol. 2017;5:154–5.
Young BE, Johnson SL, Krebs NF. Biological determinants linking infant weight gain and child obesity: current knowledge and future directions. Adv Nutr. 2012;3:675–86.
Palatianou ME, Simos YV, Andronikou SK, Kiortsis DN. Long-term metabolic effects of high birth weight: a critical review of the literature. Hormone Metabolic Res. 2014;46:911–20.
Schellong K, Schulz S, Harder T, Plagemann A. Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS ONE. 2012;7:e47776.
Acknowledgements
We gratefully thank Stephanie Winterstetter and Alexander Haag (Division of Metabolic and Nutritional Medicine, Dr von Hauner Children’s Hospital, University of Munich, Munich, Germany), who analyzed the blood plasma samples.
Funding
The authors’ work is financially supported in part by the Commission of the European Communities, Projects Early Nutrition (FP7-289346), DYNAHEALTH (H2020-633595) and LIFECYCLE (H2020-SC1-2016-RTD), and the European Research Council Advanced Grant META-GROWTH (ERC-2012-AdG 322605). No funding bodies had any role in the study design, data collection and analysis, decision to publish, or preparation of the paper.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Clinical Trial Registry number and website: ClinicalTrials.gov; identifier: NCT00338689; https://clinicaltrials.gov/ct2/show/NCT00338689.
Supplementary information
Rights and permissions
About this article
Cite this article
Kirchberg, F.F., Hellmuth, C., Totzauer, M. et al. Impact of infant protein supply and other early life factors on plasma metabolome at 5.5 and 8 years of age: a randomized trial. Int J Obes 44, 69–81 (2020). https://doi.org/10.1038/s41366-019-0398-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0398-9
This article is cited by
-
Protein, amino acids and obesity treatment
Reviews in Endocrine and Metabolic Disorders (2020)