Abstract
Background
Some weight loss medications, including liraglutide 3.0 mg, are thought to facilitate weight loss by improving appetite control. However, no studies have evaluated their long-term appetitive effects.
Subjects/methods
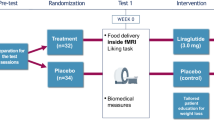
This study examined changes in appetite in a subsample of 113 adults with obesity (76.1% female, 55.8% white, BMI = 38.8 ± 4.8 kg/m2) who participated in a 52-week trial. Participants were randomized to intensive behavioral therapy alone (IBT-alone), IBT with liraglutide 3.0 mg/day (IBT-liraglutide), or IBT-liraglutide combined with a 12-week meal replacement diet (Multi-component). Participants rated their hunger, fullness after meals, liking of meals, and food preoccupation (all as experienced over the past week) using visual analogue scales (0–100 mm). Ratings were completed at baseline and eight subsequent visits over the year.
Results
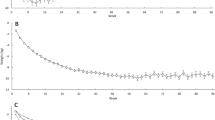
At week 52, participants treated by IBT-alone lost 6.2 ± 1.6% of baseline weight, compared with 11.8 ± 1.6% and 12.1 ± 1.5% in the IBT-liraglutide and Multi-component groups, respectively. Compared to IBT-alone, IBT-liraglutide participants reported larger reductions at week 6 in hunger (−0.3 ± 4.2 vs −16.8 ± 4.0 mm, p = .005) and food preoccupation (+0.2 ± 3.7 vs −16.3 ± 3.6 mm, p = .002) and larger increases in fullness (−5.1 ± 3.2 vs +9.8 ± 3.0 mm, p = .001). These significant differences persisted at all assessments through week 24. There were no differences between IBT-alone and IBT-liraglutide in meal liking. IBT-alone and Multi-component participants differed in hunger at week 6, and in food preoccupation at all assessments through week 24. Multi-component participants reported reduced liking of meals relative to the IBT-alone and IBT-liraglutide groups through weeks 40 and 52, respectively. There were no other differences among any groups at week 52.
Conclusions
Consistent with short-term studies, IBT-liraglutide participants reported greater improvements in hunger, fullness, and food preoccupation than those assigned to IBT-alone. Differences in appetite persisted for 24 weeks but were not maintained at week 52, despite the relatively greater weight losses in the liraglutide-treated participants at the trial’s end.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
A deidentified data set will be made available to external investigators (upon request to the first author) once the research team has completed its analysis and reporting of secondary findings from the study. This is expected to be ~2 years after the publication of this report.
References
Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–20.
Craighead LW, Stunkard AJ, O’Brien RM. Behavior therapy and pharmacotherapy for obesity. Arch Gen Psychiatry. 1981;38:763–68.
Wadden TA, Berkowitz RI, Sarwer DB, Prus-Wisniewski R, Steinberg C. Benefits of lifestyle modification in the pharmacologic treatment of obesity: a randomized trial. Arch Intern Med. 2001;161:218–27.
Phelan S, Wadden TA. Combining behavioral and pharmacological treatments for obesity. Obes Res. 2002;10:560–74.
Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016;315:2424–34.
Blundell J, Finlayson G, Axelsen M, Flint A, Gibbons C, Kvist T, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19:1242–51.
Horowitz M, Flint A, Jones KL, Hindsberger C, Rasmussen MF, Kapitza C, et al. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract. 2012;97:258–66.
Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–20.
Bagger JI, Holst JJ, Hartmann B, Andersen B, Knop FK, Vilsbøll T. Effect of Oxyntomodulin, Glucagon, GLP-1, and Combined glucagon +GLP-1 infusion on food intake, appetite, and resting energy expenditure. J Clin Endocrinol Metab. 2015;100:4541–52.
Gutzwiller J, Drewe J, Göke B, Schmidt H, Rohrer B, Lareida J, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol Regul Integr Comp Physiol. 1999;276:1541–4.
van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes. 2014;38:784–93.
Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean MEJ, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36:843–54. 2011; 2013
Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–56.
Wadden TA, Walsh OA, Berkowitz RI, Chao AM, Alamuddin N, Gruber KA, et al. Intensive behavioral therapy for obesity combined with liraglutide 3.0 mg: a randomized controlled trial. Obesity. 2019;27:75–86.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365:1969–79.
Centers for Medicare & Medicaid Services. Decision Memo for Intensive Behavioral Therapy for Obesity (CAG-00423N). 2011.
Womble LG, Wadden TA, Chandler JM, Martin AR. Agreement between weekly vs. daily assessment of appetite. Appetite. 2003;40:131–5.
Wadden TA, Stunkard AJ, Day SC, Gould RA, Rubin CJ. Less food, less hunger: reports of appetite and symptoms in a controlled study of a protein-sparing modified fast. Int J Obes. 1987;11:239–49.
Drapeau V, King N, Hetherington M, Doucet E, Blundell J, Tremblay A. Appetite sensations and satiety quotient: predictors of energy intake and weight loss. Appetite. 2007;48:159–66.
Gallop R, Tasca GA. Multilevel modeling of longitudinal data for psychotherapy researchers: II. The complexities. Psychother Res. 2009;19:438–52.
Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597–604.
MacLean PS, Bergouignan A, Cornier M, Jackman MR. Biology’s response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301:R581–R600.
Polidori D, Sanghvi A, Seeley RJ, Hall KD. How strongly does appetite counter weight loss? Quantification of the feedback control of energy intake. Obesity. 2016;24:2289–95.
Jelsing J, Vrang N, Hansen G, Raun K, Tang-Christensen M, Bjerre Knudsen L. Liraglutide: short-lived effect on gastric emptying, long lasting effects on body weight. Diabetes Obes Metab. 2012;14:531–8.
Halawi H, Khemani D, Eckert D, O'Neill J, Kadouh H, Grothe K, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol. 2017;2:890–9.
Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60:1561–5.
Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes. 2013;37:1443–51.
Marso SP, Daniels GH, Frandsen KB, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Foster GD, Wadden TA, Peterson FJ, Letizia KA, Bartlett SJ. A controlled comparison of three very-low-calorie diets: effects on weight, body composition, and symptoms. Am J Clin Nutr. 1992;55:811–7.
Acknowledgements
Trial Registration: ClinicalTrials.gov number, NCT02911818.
Funding
This work was supported by an Investigator-Initiated study grant from Novo Nordisk to TAW. JST was supported, in part, by Mentored Patient Oriented Research Award (K23DK116935) from the National Institutes of Health/National Institute of Diabetes Digestive and Kidney Disease. AMC was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number K23NR017209.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JST and NA have served as consultants to Novo Nordisk. TAW reports serving on advisory boards for Novo Nordisk and Weight Watchers Inc. RIB serves as a consultant to Eisai Pharmaceutical, and AMC has consulted with Shire Pharmaceutical. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tronieri, J.S., Wadden, T.A., Walsh, O. et al. Effects of liraglutide on appetite, food preoccupation, and food liking: results of a randomized controlled trial. Int J Obes 44, 353–361 (2020). https://doi.org/10.1038/s41366-019-0348-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0348-6
This article is cited by
-
Changes in food preferences and ingestive behaviors after glucagon-like peptide-1 analog treatment: techniques and opportunities
International Journal of Obesity (2024)
-
The Role of Lifestyle Modification with Second-Generation Anti-obesity Medications: Comparisons, Questions, and Clinical Opportunities
Current Obesity Reports (2023)
-
Beyond Weight Loss: Added Benefits Could Guide the Choice of Anti-Obesity Medications
Current Obesity Reports (2023)
-
Exploratory analysis of eating- and physical activity-related outcomes from a randomized controlled trial for weight loss maintenance with exercise and liraglutide single or combination treatment
Nature Communications (2022)
-
Effectiveness of liraglutide 3 mg for the treatment of obesity in a real-world setting without intensive lifestyle intervention
International Journal of Obesity (2021)