Abstract
Background/objectives
The cellular and extracellular matrix (ECM) interactions that regulate adipose tissue homeostasis are incompletely understood. Proteoglycans (PGs) and their sulfated glycosaminoglycans (GAGs) provide spatial and temporal signals for ECM organization and interactions with resident cells by impacting growth factor and cytokine activity. Therefore, PGs and their GAGs could be significant to adipose tissue homeostasis. The purpose of this study was to determine the role of ECM sulfated GAGs in adipose tissue homeostasis.
Methods
Adipose tissue and metabolic homeostasis in mice deficient in xylosyltransferase 2 (Xylt2−/−) were examined by histologic analyses, gene expression analyses, whole body fat composition measurements, and glucose tolerance test. Adipose tissue inflammation and adipocyte precursors were characterized by flow cytometry and in vitro culture of mesenchymal stem cells.
Results
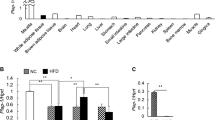
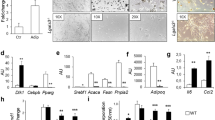
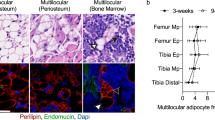
Xylt2−/− mice have low body weight due to overall reductions in abdominal fat deposition. Histologically, the adipocytes are reduced in size and number in both gonadal and mesenteric fat depots of Xylt2−/− mice. In addition, these mice are glucose intolerant, insulin resistant, and have increased serum triglycerides as compared to Xylt2 + / + control mice. Furthermore, the adipose tissue niche has increased inflammatory cells and enrichment of proinflammatory factors IL6 and IL1β, and these mice also have a loss of adipose tissue vascular endothelial cells. Lastly, xylosyltransferease-2 (XylT2) deficient mesenchymal stem cells from gonadal adipose tissue and bone marrow exhibit impaired adipogenic differentiation in vitro.
Conclusions
Decreased GAGs due to the loss of the key GAG assembly enzyme XylT2 causes reduced steady state adipose tissue stores leading to a unique lipodystrophic model. Accumulation of an adipocytic precursor pool of cells is discovered indicating an interruption in differentiation. Therefore, adipose tissue GAGs are important in the homeostasis of adipose tissue by mediating control of adipose precursor development, tissue inflammation, and vascular development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Huang G, Greenspan DS. ECM roles in the function of metabolic tissues. Trends Endocrinol Metab. 2012;23:16–22.
Kubo Y, Kaidzu S, Nakajima I, Takenouchi K, Nakamura F. Organization of extracellular matrix components during differentiation of adipocytes in long-term culture. Vitr Cell Dev Biol Anim. 2000;36:38–44.
Bouloumie A, Sengenes C, Portolan G, Galitzky J, Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes. 2001;50:2080–6.
Lijnen HR, Maquoi E, Hansen LB, Van Hoef B, Frederix L, Collen D. Matrix metalloproteinase inhibition impairs adipose tissue development in mice. Arterioscler Thromb Vasc Biol. 2002;22:374–9.
Nakajima I, Muroya S, Tanabe R, Chikuni K. Positive effect of collagen V and VI on triglyceride accumulation during differentiation in cultures of bovine intramuscular adipocytes. Differentiation. 2002;70:84–91.
Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–91.
Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983;35:657–66.
Liu J, DeYoung SM, Zhang M, Zhang M, Cheng A, Saltiel AR. Changes in integrin expression during adipocyte differentiation. Cell Metab. 2005;2:165–77.
Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–6.
Church CD, Berry R, Rodeheffer MS. Isolation and study of adipocyte precursors. Methods Enzymol. 2014;537:31–46.
Lv XJ, Zhou GD, Liu Y, Liu X, Chen JN, Luo XS, et al. In vitro proliferation and differentiation of adipose-derived stem cells isolated using anti-CD105 magnetic beads. Int J Mol Med. 2012;30:826–34.
Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–8.
Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88–e97.
Krishnan L, Hoying JB, Nguyen H, Song H, Weiss JA. Interaction of angiogenic microvessels with the extracellular matrix. Am J Physiol Heart Circ Physiol.2007;293:H3650–H3658.
Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest. 2001;108:349–55.
Hussain I, Garg A. Lipodystrophy Syndromes. Endocrinol Metab Clin North Am. 2016;45:783–97.
Nolis T. Exploring the pathophysiology behind the more common genetic and acquired lipodystrophies. J Hum Genet. 2014;59:16–23.
Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–643.
O’Reilly PJ, Hardison MT, Jackson PL, Xu X, Snelgrove RJ, Gaggar A, et al. Neutrophils contain prolyl endopeptidase and generate the chemotactic peptide, PGP, from collagen. J Neuroimmunol. 2009;217:51–54.
Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–33.
Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhao JG, et al. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal. 2011;4:ra75.
Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–9.
Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20:9–22.
Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–21.
Esko JD, Kimata, K, and Lindahl, U Proteoglycans and Sulfated Glycosaminoglycans. Essentials of glycobiology. 2nd ed. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, N.Y; 2009.
Cuellar K, Chuong H, Hubbell SM, Hinsdale ME. Biosynthesis of chondroitin and heparan sulfate in Chinese hamster ovary cells depends on xylosyltransferase II. J Biol Chem. 2007;282:5195–5200.
Condac E, Silasi-Mansat R, Kosanke S, Schoeb T, Towner R, Lupu F, et al. Polycystic disease caused by deficiency in xylosyltransferase 2, an initiating enzyme of glycosaminoglycan biosynthesis. Proc Natl Acad Sci USA. 2007;104:9416–21.
Gotting C, Kuhn J, Zahn R, Brinkmann T, Kleesiek K. Molecular cloning and expression of human UDP-d-Xylose:proteoglycan core protein beta-d-xylosyltransferase and its first isoform XT-II. J Mol Biol. 2000;304:517–28.
Roch C, Kuhn J, Kleesiek K, Gotting C. Differences in gene expression of human xylosyltransferases and determination of acceptor specificities for various proteoglycans. Biochem Biophys Res Commun. 2010;391:685–91.
Bolton K, Segal D, McMillan J, Jowett J, Heilbronn L, Abberton K, et al. Decorin is a secreted protein associated with obesity and type 2 diabetes. Int J Obes (Lond). 2008;32:1113–21.
Bolton K, Segal D, Walder K. The small leucine-rich proteoglycan, biglycan, is highly expressed in adipose tissue of Psammomys obesus and is associated with obesity and type 2 diabetes. Biologics. 2012;6:67–72.
Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–37.
Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102–6.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif. 2001;25:402–8.
Liu D, Wang CJ, Judge DP, Halushka MK, Ni J, Habashi JP, et al. A Pkd1-Fbn1 genetic interaction implicates TGF-beta signaling in the pathogenesis of vascular complications in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2014;25:81–91.
Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res. 2009;50:S86–S90.
Huang ZH, Minshall RD, Mazzone T. Mechanism for endogenously expressed ApoE modulation of adipocyte very low density lipoprotein metabolism: role in endocytic and lipase-mediated metabolic pathways. J Biol Chem. 2009;284:31512–22.
Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–56.
Zamani N, Brown CW. Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocr Rev. 2011;32:387–403.
Zhou S, Eid K, Glowacki J. Cooperation between TGF-beta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res. 2004;19:463–70.
Longo KA, Wright WS, Kang S, Gerin I, Chiang SH, Lucas PC, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279:35503–9.
Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–3.
Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–26.
Hager G, Holnthoner W, Wolbank S, Husa AM, Godthardt K, Redl H, et al. Three specific antigens to isolate endothelial progenitor cells from human liposuction material. Cytotherapy. 2013;15:1426–35.
Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–63.
Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–9.
Lee SW, Jeong HK, Lee JY, Yang J, Lee EJ, Kim SY, et al. Hypoxic priming of mESCs accelerates vascular-lineage differentiation through HIF1-mediated inverse regulation of Oct4 and VEGF. EMBO Mol Med. 2012;4:924–38.
Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol. 2011;7:137–50.
Birsoy K, Berry R, Wang T, Ceyhan O, Tavazoie S, Friedman JM, et al. Analysis of gene networks in white adipose tissue development reveals a role for ETS2 in adipogenesis. Development. 2011;138:4709–19.
Cautivo KM, Lizama CO, Tapia PJ, Agarwal AK, Garg A, Horton JD, et al. AGPAT2 is essential for postnatal development and maintenance of white and brown adipose tissue. Mol Metab. 2016;5:491–505.
Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Development. 2013;140:3939–49.
Dubois SG, Heilbronn LK, Smith SR, Albu JB, Kelley DE, Ravussin E, et al. Decreased expression of adipogenic genes in obese subjects with type 2 diabetes. Obes (Silver Spring). 2006;14:1543–52.
Yang X, Jansson PA, Nagaev I, Jack MM, Carvalho E, Sunnerhagen KS, et al. Evidence of impaired adipogenesis in insulin resistance. Biochem Biophys Res Commun. 2004;317:1045–51.
Herrero L, Shapiro H, Nayer A, Lee J, Shoelson SE. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc Natl Acad Sci USA. 2010;107:240–5.
Teran M, Nugent MA. Synergistic binding of vascular endothelial growth factor-A and its receptors to heparin selectively modulates complex affinity. J Biol Chem. 2015;290:16451–62.
Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–8.
Aikio M, Elamaa H, Vicente D, Izzi V, Kaur I, Seppinen L, et al. Specific collagen XVIII isoforms promote adipose tissue accrual via mechanisms determining adipocyte number and affect fat deposition. Proc Natl Acad Sci USA. 2014;111:E3043–3052.
Acknowledgements
This work was supported by funds from the Oklahoma Center for Adult Stem Cell Research, a program of TSET, NIH Center of Biomedical Research Excellence (COBRE), P20 GM103648, P20GM103441, P30GM114731, Oklahoma State University Research Activity funding. We also thank the COBRE Animal Models Core for assistance. We also thank Huan Song for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sivasami, P., Poudel, N., Munteanu, M.C. et al. Adipose tissue loss and lipodystrophy in xylosyltransferase II deficient mice. Int J Obes 43, 1783–1794 (2019). https://doi.org/10.1038/s41366-019-0324-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0324-1
This article is cited by
-
Enlarged adipocytes from subcutaneous vs. visceral adipose tissue differentially contribute to metabolic dysfunction and atherogenic risk of patients with obesity
Scientific Reports (2021)
-
Xylosyltransferase 2 deficiency and organ homeostasis
Glycoconjugate Journal (2020)