Abstract
Objective
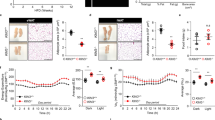
Translin knockout (KO) mice display robust adiposity. Recent studies indicate that translin and its partner protein, trax, regulate the microRNA and ATM kinase signaling pathways, both of which have been implicated in regulating metabolism. In the course of characterizing the metabolic profile of these mice, we found that they display normal glucose tolerance despite their elevated adiposity. Accordingly, we investigated why translin KO mice display this paradoxical phenotype.
Methods
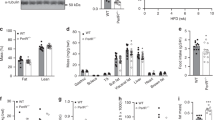
To help distinguish between the metabolic effects of increased adiposity and those of translin deletion per se, we compared three groups: (1) wild-type (WT), (2) translin KO mice on a standard chow diet, and (3) adiposity-matched WT mice that were placed on a high-fat diet until they matched translin KO adiposity levels. All groups were scanned to determine their body composition and tested to evaluate their glucose and insulin tolerance. Plasma, hepatic, and adipose tissue samples were collected and used for histological and molecular analyses.
Results
Translin KO mice show normal glucose tolerance whereas adiposity-matched WT mice, placed on a high-fat diet, do not. In addition, translin KO mice display prominent hepatic steatosis that is more severe than that of adiposity-matched WT mice. Unlike adiposity-matched WT mice, translin KO mice display three key features that have been shown to reduce susceptibility to insulin resistance: increased accumulation of subcutaneous fat, increased levels of circulating adiponectin, and decreased Tnfα expression in hepatic and adipose tissue.
Conclusions
The ability of translin KO mice to retain normal glucose tolerance in the face of marked adipose tissue expansion may be due to the three protective factors noted above. Further studies aimed at defining the molecular bases for this combination of protective phenotypes may yield new approaches to limit the adverse metabolic consequences of obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Brommage R, Desai U, Revelli JP, Donoviel DB, Fontenot GK, Dacosta CM, et al. High-throughput screening of mouse knockout lines identifies true lean and obese phenotypes. Obesity (Silver Spring). 2008;16:2362–7.
Chennathukuzhi V, Stein JM, Abel T, Donlon S, Yang S, Miller JP, et al. Mice deficient for testis-brain RNA-binding protein exhibit a coordinate loss of TRAX, reduced fertility, altered gene expression in the brain, and behavioral changes. Mol Cell Biol. 2003;23:6419–34.
Asada K, Canestrari E, Fu X, Li Z, Makowski E, Wu YC, et al. Rescuing dicer defects via inhibition of an anti-dicing nuclease. Cell Rep. 2014;9:1471–81.
Baraban JM, Shah A, Fu X. Multiple pathways mediate microRNA degradation: focus on the translin/trax RNase complex. Adv Pharmacol. 2018;82:1–20.
Wang JY, Chen SY, Sun CN, Chien T, Chern Y. A central role of TRAX in the ATM-mediated DNA repair. Oncogene. 2016;35:1657–70.
Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol. 2000;2:893–8.
Fukuda Y, Ishida R, Aoki K, Nakahara K, Takashi T, Mochida K, et al. Contribution of translin to hematopoietic regeneration after sublethal ionizing irradiation. Biol Pharm Bull. 2008;31:207–11.
Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–87.
Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, et al. A guide to analysis of mouse energy metabolism. Nat Methods. 2011;9:57–63.
Galarraga M, Campion J, Munoz-Barrutia A, Boque N, Moreno H, Martinez JA, et al. Adiposoft: automated software for the analysis of white adipose tissue cellularity in histological sections. J Lipid Res. 2012;53:2791–6.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82.
Scott KA, Yamazaki Y, Yamamoto M, Lin Y, Melhorn SJ, Krause EG, et al. Glucose parameters are altered in mouse offspring produced by assisted reproductive technologies and somatic cell nuclear transfer. Biol Reprod. 2010;83:220–7.
Kayser BD, Goran MI, Bouret SG. Perinatal overnutrition exacerbates adipose tissue inflammation caused by high-fat feeding in C57BL/6J mice. PLoS ONE. 2015;10:e0121954.
Finkenstadt PM, Kang WS, Jeon M, Taira E, Tang W, Baraban JM. Somatodendritic localization of translin, a component of the translin/trax RNA binding complex. J Neurochem. 2000;75:1754–62.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Colak Y, Ozturk O, Senates E, Tuncer I, Yorulmaz E, Adali G, et al. SIRT1 as a potential therapeutic target for treatment of nonalcoholic fatty liver disease. Med Sci Monit. 2011;17:HY5–9.
Li Z, Wu Y, Baraban JM. The Translin/Trax RNA binding complex: clues to function in the nervous system. Biochim Biophys Acta. 2008;1779:479–85.
Kammoun HL, Kraakman MJ, Febbraio MA. Adipose tissue inflammation in glucose metabolism. Rev Endocr Metab Disord. 2014;15:31–44.
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83.
Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239.
Asterholm IW, Scherer PE. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am J Pathol. 2010;176:1364–76.
Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53.
Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CcC, Itani SI, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA. 2002;99:16309–13.
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6.
Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–85.
Kasai M, Ishida R, Nakahara K, Okumura K, Aoki K. Mesenchymal cell differentiation and diseases: involvement of translin/TRAX complexes and associated proteins. Ann N Y Acad Sci. 2018;1421:37–45
Li Z, Hardij J, Bagchi DP, Scheller EL, MacDougald OA. Development, regulation, metabolism and function of bone marrow adipose tissues. Bone. 2018;110:134–40.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84.
Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23.
Ouchi N, Walsh K. A novel role for adiponectin in the regulation of inflammation. Arterioscler Thromb Vasc Biol. 2008;28:1219–21.
Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7.
Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100.
Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–7.
Karastergiou K, Fried SK. Cellular mechanisms driving sex differences in adipose tissue biology and body shape in humans and mouse models. Adv Exp Med Biol. 2017;1043:29–51.
Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–9.
Ikeuchi Y, Imanishi A, Sudo K, Fukunaga T, Yokoi A, Matsubara L, et al. Translin modulates mesenchymal cell proliferation and differentiation in mice. Biochem Biophys Res Commun. 2018;504:115–22.
Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91.
Ding RB, Bao J, Deng CX. Emerging roles of SIRT1 in fatty liver diseases. Int J Biol Sci. 2017;13:852–67.
Schug TT, Li X. Sirtuin 1 in lipid metabolism and obesity. Ann Med. 2011;43:198–211.
Kemper JK, Choi SE, Kim DH. Sirtuin 1 deacetylase: a key regulator of hepatic lipid metabolism. Vitam Horm. 2013;91:385–404.
Chartoumpekis DV, Zaravinos A, Ziros PG, Iskrenova RP, Psyrogiannis AI, Kyriazopoulou VE, et al. Differential expression of microRNAs in adipose tissue after long-term high-fat diet-induced obesity in mice. PLoS ONE. 2012;7:e34872.
Nakanishi N, Nakagawa Y, Tokushige N, Aoki N, Matsuzaka T, Ishii K, et al. The up-regulation of microRNA-335 is associated with lipid metabolism in liver and white adipose tissue of genetically obese mice. Biochem Biophys Res Commun. 2009;385:492–6.
Oger F, Gheeraert C, Mogilenko D, Benomar Y, Molendi-Coste O, Bouchaert E, et al. Cell-specific dysregulation of microRNA expression in obese white adipose tissue. J Clin Endocrinol Metab. 2014;99:2821–33.
Tome M, Lopez-Romero P, Albo C, Sepulveda JC, Fernandez-Gutierrez B, Dopazo A, et al. miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ. 2011;18:985–95.
Chou CH, Shrestha S, Yang CD, Chang NW, Lin YL, Liao KW, et al. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46:D296–D302.
Harley IT, Stankiewicz TE, Giles DA, Softic S, Flick LM, Cappelletti M, et al. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology. 2014;59:1830–9.
Shin JH, Shin DW, Noh M. Interleukin-17A inhibits adipocyte differentiation in human mesenchymal stem cells and regulates pro-inflammatory responses in adipocytes. Biochem Pharmacol. 2009;77:1835–44.
Chern Y, Chien T, Fu X, Shah AP, Abel T, Baraban JM. Trax: a versatile signaling protein plays key roles in synaptic plasticity and DNA repair. Neurobiol Learn Mem. 2018.
Hasegawa J, Osatomi K, Wu RF, Uyeda K. A novel factor binding to the glucose response elements of liver pyruvate kinase and fatty acid synthase genes. J Biol Chem. 1999;274:1100–7.
Wu RF, Osatomi K, Terada LS, Uyeda K. Identification of translin/trax complex as a glucose response element binding protein in liver. Biochim Biophys Acta. 2003;1624:29–35.
Acknowledgements
The authors thank Dr. Timothy H. Moran (Johns Hopkins University) for reviewing the manuscript and Dr. Andrew Wolfe for assistance with the indirect calorimetric measurements. We thank Ginny Miller, Zachary Cordner, Seva Khambadkone, and Leonard Marque for their technical assistance. We also thank Conovor Talbot Jr. (the Johns Hopkins Deep Sequencing and Microarray Core Facility) for the microarray analyses, Michele Pucak (the Johns Hopkins Department of Neuroscience Multiphoton Imaging Core), and Nadine Forbes-McBean (the Johns Hopkins Phenotyping Core). Histological procedures were performed by The Johns Hopkins Medical Institutions Reference Histology Laboratory. This work was supported by an NIH/NIDA grant, DA00266 and NINDS grant NS050274.
Author information
Authors and Affiliations
Contributions
APS, MDJ, and XF contributed equally to this work. APS, MDJ, XF, and GJB performed the research. APS, MDJ, XF, GJB, and MS analyzed the data. APS, MDJ, XF, GJB, MJW, KLT, and JMB designed the experiments. APS, MDJ, XF, KLT, and JMB drafted the manuscript. GJB and MJW helped revise the manuscript. APS, MDJ, XF, GJB, and KLT, and JMB are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, A.P., Johnson, M.D., Fu, X. et al. Deletion of translin (Tsn) induces robust adiposity and hepatic steatosis without impairing glucose tolerance. Int J Obes 44, 254–266 (2020). https://doi.org/10.1038/s41366-018-0315-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0315-7
This article is cited by
-
Elevated body fat increases amphetamine accumulation in brain: evidence from genetic and diet-induced forms of adiposity
Translational Psychiatry (2021)