Abstract
Background and aims
Prepregnancy maternal obesity is a global health problem and has been associated with offspring metabolic and mental ill-health. However, there is a knowledge gap in understanding potential neurobiological factors related to these associations. This study explored the relation between maternal prepregnancy body mass index (BMI) and offspring brain white matter microstructure at the age of 6, 10, and 26 years in three independent cohorts.
Subjects and methods
The study used data from three European birth cohorts (n = 116 children aged 6 years, n = 2466 children aged 10 years, and n = 437 young adults aged 26 years). Information on maternal prepregnancy BMI was obtained before or during pregnancy and offspring brain white matter microstructure was measured at age 6, 10, or 26 years. We used magnetic resonance imaging-derived fractional anisotropy (FA) and mean diffusivity (MD) as measures of white matter microstructure in the brainstem, callosal, limbic, association, and projection tracts. Linear regressions were fitted to examine the association of maternal BMI and offspring white matter microstructure, adjusting for several socioeconomic and lifestyle-related confounders, including education, smoking, and alcohol use.
Results
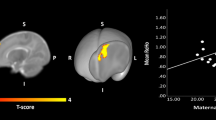
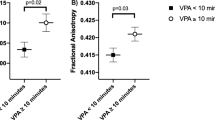
Maternal BMI was associated with higher FA and lower MD in multiple brain tracts, for example, association and projection fibers, in offspring aged 10 and 26 years, but not at 6 years. In each cohort maternal BMI was related to different white matter tract and thus no common associations across the cohorts were found.
Conclusions
Maternal BMI was associated with higher FA and lower MD in multiple brain tracts in offspring aged 10 and 26 years, but not at 6 years of age. Future studies should examine whether our observations can be replicated and explore the potential causal nature of the findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29–e36.
Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–6.
Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347:f4539.
Basatemur E, Gardiner J, Williams C, Melhuish E, Barnes J, Sutcliffe A. Maternal prepregnancy BMI and child cognition: a longitudinal cohort study. Pediatrics. 2013;131:56–63.
Bliddal M, Olsen J, Støvring H, Eriksen H-LF, Kesmodel US, TIA Sørensen, et al. Maternal pre-pregnancy BMI and intelligence quotient (IQ) in 5-year-old children: a cohort based study. PLoS ONE. 2014;9:e94498.
Heikura U, Taanila A, Hartikainen A-L, Olsén P, Linna S-L, Wendt Lvon, et al. Variations in prenatal sociodemographic factors associated with intellectual disability: a study of the 20-year interval between two birth cohorts in Northern Finland. Am J Epidemiol. 2008;167:169–77.
Hinkle SN, Schieve LA, Stein AD, Swan DW, Ramakrishnan U, Sharma AJ. Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age. Int J Obes. 2012;36:1312–9.
Huang L, Yu X, Keim S, Li L, Zhang L, Zhang J. Maternal prepregnancy obesity and child neurodevelopment in the Collaborative Perinatal Project. Int J Epidemiol. 2014;43:783–92.
Widen EM, Kahn LG, Cirillo P, Cohn B, Kezios KL, Factor-Litvak P. Prepregnancy overweight and obesity are associated with impaired child neurodevelopment. Matern Child Nutr. 2018;14:n/a–n/a.
Yeung EH, Sundaram R, Ghassabian A, Xie Y, Louis GB. Parental obesity and early childhood development. Pediatrics. 2017;139:e20161459.
Mina TH, Lahti M, Drake AJ, Denison FC, Räikkönen K, Norman JE, et al. Prenatal exposure to maternal very severe obesity is associated with impaired neurodevelopment and executive functioning in children. Pediatr Res. 2017;82:47–54.
Rodriguez A. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J Child Psychol Psychiatry. 2010;51:134–43.
Deardorff J, Smith LH, Petito L, Kim H, Abrams BF. Maternal prepregnancy weight and children’s behavioral and emotional outcomes. Am J Prev Med. 2017;53:432–40.
Adane AA, Mishra GD, Tooth LR. Maternal pre-pregnancy obesity and childhood physical and cognitive development of children: a systematic review. Int J Obes. 2016;40:1608–18.
Li X, Andres A, Shankar K, Pivik RT, Glasier CM, Ramakrishnaiah RH, et al. Differences in brain functional connectivity at resting state in neonates born to healthy obese or normal-weight mothers. Int J Obes. 2016;40:1931–4.
Ou X, Thakali KM, Shankar K, Andres A, Badger TM. Maternal adiposity negatively influences infant brain white matter development. Obesity. 2015;23:1047–54.
Campoy C, Martín-Bautista E, García-Valdés L, Florido J, Agil A, Lorente JA, et al. Study of maternal nutrition and genetic on the foetal adiposity programming: The PREOBE study. Nutr Hosp. 2008;23:584–90.
Kooijman MN, Kruithof CJ, Duijn CM, van, Duijts L, Franco OH, van IJzendoorn MH. et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31:1243–64.
White T, Muetzel RL, Marroun HE, Blanken LME, Jansen P, Bolhuis K, et al. Paediatric population neuroimaging and the Generation R Study: the second wave. Eur J Epidemiol. 2018;33:99–125.
Riitta JM, Anna‐Liisa H‐S, Paula R. Labour induction policy in hospitals of different levels of specialisation. Int J Obstet Gynaecol. 2005;100:310–5.
Björnholm L, Nikkinen J, Kiviniemi V, Nordström T, Niemelä S, Drakesmith M, et al. Structural properties of the human corpus callosum: multimodal assessment and sex differences. Neuroimage. 2017;152:108–18.
Olsén P, Läärä E, Rantakallio P, Järvelin M-R, Sarpola A, Hartikainen A-L. Epidemiology of preterm delivery in two birth cohorts with an interval of 20 years. Am J Epidemiol. 1995;142:1184–93.
Muetzel RL, Blanken LME, van der Ende J, El Marroun H, Shaw P, Sudre G. et al. Tracking brain evelopment and dimensional psychiatric symptoms in hildren: a longitudinal population-based neuroimaging study. AJP. 2017;175:54–62.
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–90.
Haselgrove JC, Moore JR. Correction for distortion of echo-planar images used to calculate the apparent diffusion coefficient. Magn Reson Med. 1996;36:960–4.
Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55.
Groot M, de Ikram MA, Akoudad S, Krestin GP, Hofman A, Lugt Avander. et al. Tract-specific white matter degeneration in aging: The Rotterdam Study. Alzheimer’s Dement. 2015;11:321–30.
Gaillard R, Welten M, Oddy WH, Beilin LJ, Mori TA, Jaddoe VWV et al. Associations of maternal prepregnancy body mass index and gestational weight gain with cardio-metabolic risk factors in adolescent offspring: a prospective cohort study. BJOG 2016;123: 207–16.
Jharap VV, Santos S, Steegers EAP, Jaddoe VWV, Gaillard R. Associations of maternal obesity and excessive weight gain during pregnancy with subcutaneous fat mass in infancy. Early Hum Dev. 2017;108:23–28.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol). 1995;57:289–300.
R Core Team (2018). R: A language and environment for statistical computing. R foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Aubert-Broche B, Fonov V, Leppert I, Pike GB, Collins DL. Human brain myelination from birth to 4.5 years. Med Image Comput Comput Assist Interv. 2008;11:180–7.
Carnell S, Benson L, Chang K-Y (Virginia), Wang Z, Huo Y, Geliebter A. et al. Neural correlates of familial obesity risk and overweight in adolescence. Neuroimage. 2017;159:236–47.
Hayden BY, Platt ML. Neurons in anterior cingulate cortex multiplex information about reward and action. J Neurosci. 2010;30:3339–46.
LaMantia AS, Rakic P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J Neurosci. 1990;10:2156–75.
LaMantia AS, Rakic P. Axon overproduction and elimination in the anterior commissure of the developing rhesus monkey. J Comp Neurol. 1994;340:328–36.
Paus T. Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn. 2010;72:26–35.
Graf AE, Haines KM, Pierson CR, Bolon BN, Houston RH, Velten M, et al. Perinatal inflammation results in decreased oligodendrocyte numbers in adulthood. Life Sci. 2014;94:164–71.
Madan JC, Davis JM, Craig WY, Collins M, Allan W, Quinn R, et al. Maternal obesity and markers of inflammation in pregnancy. Cytokine. 2009;47:61–64.
Burg JW, van der, Sen S, Chomitz VR, Seidell JC, Leviton A, Dammann O. The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatr Res. 2015;79:3–12.
Graf AE, Lallier SW, Waidyaratne G, Thompson MD, Tipple TE, Hester ME, et al. Maternal high fat diet exposure is associated with increased hepcidin levels, decreased myelination, and neurobehavioral changes in male offspring. Brain Behav Immun. 2016;58:369–78.
White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim S-O, Hise TL, et al. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis. 2009;35:3–13.
Bergmann S, Schlesier-Michel A, Wendt V, Grube M, Keitel-Korndörfer A, Gausche R et al. Maternal weight predicts children’s psychosocial development via parenting stress and emotional availability. Front Psychol. 2016; 7:1156.
Panagos PG, Vishwanathan R, Penfield-Cyr A, Matthan NR, Shivappa N, Wirth MD, et al. Breastmilk from obese mothers has pro-inflammatory properties and decreased neuroprotective factors. J Perinatol. 2016;36:284–90.
Edlow A, Hui L, Wick H, Fried I, Bianchi D. Assessing the fetal effects of maternal obesity via transcriptomic analysis of cord blood: a prospective case–control study. BJOG. 2016;123:180–9.
Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–52.
Keim SA, Branum AM, Klebanoff MA, Zemel BS. Maternal body mass index and daughters’ age at menarche. Epidemiology. 2009;20:677–81.
Birdsill AC, Oleson S, Kaur S, Pasha E, Ireton A, Tanaka H, et al. Abdominal obesity and white matter microstructure in midlife. Hum Brain Mapp. 2017;38:3337–44.
Ong ZY, Muhlhausler BS. Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J. 2011;25:2167–79.
Stachowiak EK, Srinivasan M, Stachowiak MK, Patel MS. Maternal obesity induced by a high fat diet causes altered cellular development in fetal brains suggestive of a predisposition of offspring to neurological disorders in later life. Metab Brain Dis. 2013;28:721–5.
Williams L, Seki Y, Vuguin PM, Charron MJ. Animal models of in utero exposure to a high fat diet: a review. Biochim Biophys Acta. 2014;1842:507–19.
Acknowledgements
This work was supported by the European Union’s Horizon 2020 research and innovation program [grant agreement no. 633595 DynaHEALTH] and no. 733206 LifeCycle], the Netherlands Organization for Health Research and Development [ZONMW Vici project 016.VICI.170.200]. The PREOBE cohort was funded by Spanish Ministry of Innovation and Science. Junta de Andalucía: Excellence Projects (P06-CTS-02341) and Spanish Ministry of Economy and Competitiveness (BFU2012-40254-C03-01). The first phase of the Generation R Study is made possible by financial support from the Erasmus Medical Centre, the Erasmus University, and the Netherlands Organization for Health Research and Development (ZonMW, grant ZonMW Geestkracht 10.000.1003). The Northern Finland Birth Cohort 1986 is funded by University of Oulu, University Hospital of Oulu, Academy of Finland (EGEA), Sigrid Juselius Foundation, European Commission (EURO-BLCS, Framework 5 award QLG1-CT-2000-01643), NIH/NIMH (5R01MH63706:02)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Verdejo-Román, J., Björnholm, L., Muetzel, R.L. et al. Maternal prepregnancy body mass index and offspring white matter microstructure: results from three birth cohorts. Int J Obes 43, 1995–2006 (2019). https://doi.org/10.1038/s41366-018-0268-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0268-x
This article is cited by
-
Associations between maternal pre-pregnancy BMI and infant striatal mean diffusivity
BMC Medicine (2024)
-
Episodic memory dysfunction and hypersynchrony in brain functional networks in cognitively intact subjects and MCI: a study of 379 individuals
GeroScience (2023)
-
The impact of maternal obesity on childhood neurodevelopment
Journal of Perinatology (2021)
-
Maternal body mass index in pregnancy and mental disorders in adult offspring: a record linkage study in Aberdeen, Scotland
Scientific Reports (2021)
-
Structural Alterations in Large-scale Brain Networks and Their Relationship with Sleep Disturbances in the Adolescent Population
Scientific Reports (2020)