Abstract
Background/objectives:
Ascorbic acid is a known cofactor in the biosynthesis of carnitine, a molecule that has an obligatory role in fatty acid oxidation. Our previous studies have demonstrated that obesity is regulated effectively through peroxisome proliferator-activated receptor α (PPARα)-mediated fatty acid β-oxidation. Thus, this study aimed to determine whether ascorbic acid can inhibit obesity and nonalcoholic fatty liver disease (NAFLD) in part through the actions of PPARα.
Design:
After C57BL/6J mice received a low-fat diet (LFD, 10% kcal fat), a high-fat diet (HFD, 45% kcal fat), or the same HFD supplemented with ascorbic acid (1% w/w) (HFD-AA) for 15 weeks, variables and determinants of visceral obesity and NAFLD were examined using metabolic measurements, histology, and gene expression.
Results:
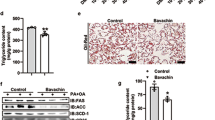
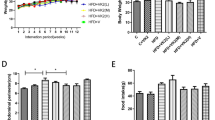
Compared to HFD-fed obese mice, administration of HFD-AA to obese mice reduced body weight gain, visceral adipose tissue mass, and visceral adipocyte size without affecting food consumption profiles. Concomitantly, circulating ascorbic acid concentrations were significantly higher in HFD-AA mice than in HFD mice. Ascorbic acid supplementation increased the mRNA levels of PPARα and its target enzymes involved in fatty acid β-oxidation in visceral adipose tissues. Consistent with the effects of ascorbic acid on visceral obesity, ascorbic acid not only inhibited hepatic steatosis but also increased the mRNA levels of PPARα-dependent fatty acid β-oxidation genes in livers. Similarly, hepatic inflammation, fibrosis, and apoptosis were also decreased during ascorbic acid-induced inhibition of visceral obesity. In addition, serum levels of alanine aminotransferase, aspartate aminotransferase, total cholesterol, and LDL cholesterol were lower in HFD-AA-fed mice than in those of HFD-fed mice.
Conclusions:
These results suggest that ascorbic acid seems to suppress HFD-induced visceral obesity and NAFLD in part through the activation of PPARα.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am Heart J. 2005;149:33–45.
Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation. 2003;108:1546–51.
Rogge MM. The role of impaired mitochondrial lipid oxidation in obesity. Biol Res Nurs. 2009;10:356–73.
Yoon M. PPARα in obesity: sex difference and estrogen involvement. PPAR Res. 2010;2010:584296.
Yoon M. The role of PPARα in lipid metabolism and obesity: focusing on the effects of estrogen on PPARα actions. Pharmacol Res. 2009;60:151–9.
Liang CP, Tall AR. Transcriptional profiling reveals global defects in energy metabolism, lipoprotein, and bile acid synthesis and transport with reversal by leptin treatment in ob/ob mouse liver. J Biol Chem. 2001;276:49066–76.
Jeong S, Kim M, Han M, Lee H, Ahn J, Kim M, et al. Fenofibrate prevents obesity and hypertriglyceridemia in low-density lipoprotein receptor-null mice. Metabolism. 2004;53:607–13.
Jeong S, Yoon M. Fenofibrate inhibits adipocyte hypertrophy and insulin resistance by activating adipose PPARα in high fat diet-induced obese mice. Exp Mol Med. 2009;41:397–405.
Johnston CS, Beezhold BL, Mostow B, Swan PD. Plasma vitamin C is inversely related to body mass index and waist circumference but not to plasma adiponectin in nonsmoking adults. J Nutr. 2007;137:1757–62.
da Silva VR, Moreira EA, Wilhelm-Filho D, de Miranda JX, Benincá JP, Vigil SV, et al. Proinflammatory and oxidative stress markers in patients submitted to Roux-en-Y gastric bypass after 1 year of follow-up. Eur J Clin Nutr. 2012;66:891–9.
Johnston CS. Strategies for healthy weight loss: from vitamin C to the glycemic response. J Am Coll Nutr. 2005;24:158–65.
Canoy D, Wareham N, Welch A, Bingham S, Luben R, Day N, et al. Plasma ascorbic acid concentrations and fat distribution in 19,068 British men and women in the European Prospective Investigation into Cancer and Nutrition Norfolk cohort study. Am J Clin Nutr. 2005;82:1203–9.
Johnston CS, Corte C, Swan PD. Marginal vitamin C status is associated with reduced fat oxidation during submaximal exercise in young adults. Nutr Metab. 2006;3:35.
Naylor GJ, Grant L, Smith C. A double blind placebo controlled trial of ascorbic acid in obesity. Nutr Health. 1985;4:25–8.
Garcia-Diaz DF, Lopez-Legarrea P, Quintero P, Martinez JA. Vitamin C in the treatment and/or prevention of obesity. J Nutr Sci Vitaminol. 2014;60:367–79.
Campión J, Milagro FI, Fernández D, Martínez JA. Diferential gene expression and adiposity reduction induced by ascorbic acid supplementation in a cafeteria model of obesity. J Physiol Biochem. 2006;62:71–80.
Kim B, Choi KM, Yim HS, Park HT, Yim JH, Lee MG. Adipogenic and lipolytic effects of ascorbic acid in ovariectomized rats. Yonsei Med J. 2018;59:85–91.
Abdel-Wahab YH, O’Harte FP, Mooney MH, Barnett CR, Flatt PR. Vitamin C supplementation decreases insulin glycation and improves glucose homeostasis in obese hyperglycemic (ob/ob) mice. Metabolism. 2002;51:514–7.
Oliveira CP, Gayotto LC, Tatai C, Della Nina BI, Lima ES, Abdalla DS, et al. Vitamin C and vitamin E in prevention of nonalcoholic fatty liver disease (NAFLD) in choline deficient diet fed rats. Nutr J. 2003;2:9.
Hoppel C. The role of carnitine in normal and altered fatty acid metabolism. Am J Kidney Dis. 2003;41:S4–12.
Tsao CS, Leung PY, Young M. Effect of dietary ascorbic acid intake on tissue vitamin C in mice. J Nutr. 1987;117:291–7.
Wilson JX. The physiological role of dehydroascorbic acid. FEBS Lett. 2002;527:5–9.
Chatterjee IB, Majumder AK, Nandi BK, Subramanian N. Synthesis and some major functions of vitamin C in animals. Ann N Y Acad Sci. 1975;258:24–47.
Grollman AP, Lehninger AL. Enzymic synthesis of L-ascorbic acid in different animal species. Arch Biochem Biophys. 1957;69:458–67.
Donpunha W, Kukongviriyapan U, Sompamit K, Pakdeechote P, Kukongviriyapan V, Pannangpetch P. Protective effect of ascorbic acid on cadmium-induced hypertension and vascular dysfunction in mice. Biometals. 2011;24:105–15.
Thornton SJ, Wong IT, Neumann R, Kozlowski P, Wasan KM. Dietary supplementation with phytosterol and ascorbic acid reduces body mass accumulation and alters food transit time in a diet-induced obesity mouse model. Lipids Health Dis. 2011;10:107.
Bannerman B, Xu L, Jones M, Tsu C, Yu J, Hales P, et al. Preclinical evaluation of the antitumor activity of bortezomib in combination with vitamin C or with epigallocatechin gallate, a component of green tea. Cancer Chemother Pharmacol. 2011;68:1145–54.
Lyons JA, Haring JS, Biga PR. Myostatin expression, lymphocyte population, and potential cytokine production correlate with predisposition to high-fat diet induced obesity in mice. PLoS ONE. 2010;5:e12928.
Kim J, Lee H, Lim J, Oh J, Shin SS, Yoon M. The angiogenesis inhibitor ALS-L1023 from lemon-balm leaves attenuates high-fat diet-induced nonalcoholic fatty liver disease through regulating the visceral adipose-tissue function. Int J Mol Sci. 2017;18:E846.
Hansen HH, Feigh M, Veidal SS, Rigbolt KT, Vrang N, Fosgerau K. Mouse models of nonalcoholic steatohepatitis in preclinical drug development. Drug Discov Today. 2017;22:1707–18.
Santhekadur PK, Kumar DP, Sanyal AJ. Preclinical models of non-alcoholic fatty liver disease. J Hepatol. 2018;68:230–7.
Kant AK. Interaction of body mass index and attempt to lose weight in a national sample of US adults: association with reported food and nutrient intake, and biomarkers. Eur J Clin Nutr. 2003;57:249–59.
Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7.
Blaak EE. Fatty acid metabolism in obesity and type 2 diabetes mellitus. Proc Nutr Soc. 2003;62:753–60.
Yoon S, Kim J, Lee H, Lee H, Lim J, Yang H, et al. The effects of herbal composition Gambigyeongsinhwan (4) on hepatic steatosis and inflammation in Otsuka Long-Evans Tokushima fatty rats and HepG2 cells. J Ethnopharmacol. 2017;195:204–13.
Shin SS, Jung YS, Yoon KH, Choi S, Hong Y, Park D, et al. The Korean traditional medicine gyeongshingangjeehwan inhibits adipocyte hypertrophy and visceral adipose tissue accumulation by activating PPARalpha actions in rat white adipose tissues. J Ethnopharmacol. 2010;127:47–54.
Roh JS, Lee H, Woo S, Yoon M, Kim J, Park SD, et al. Herbal composition Gambigyeongsinhwan (4) from Curcuma longa, Alnus japonica, and Massa Medicata Fermentata inhibits lipid accumulation in 3T3-L1 cells and regulates obesity in Otsuka Long-Evans Tokushima Fatty rats. J Ethnopharmacol. 2015;171:287–94.
van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008;94:231–41.
Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–51.
Labbe G, Pessayre D, Fromenty B. Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol. 2008;22:335–53.
Takahashi Y, Fukusato T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:15539–48.
Chalasani N, Wilson L, Kleiner DE, Cummings OW, Brunt EM, Unalp A. et al. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. J Hepatol. 2008;48:829–34.
Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371–9.
Chatzigeorgiou A, Chung KJ, Garcia-Martin R, Alexaki VI, Klotzsche-von Ameln A, Phieler J, et al. Dual role of B7 costimulation in obesity-related nonalcoholic steatohepatitis and metabolic dysregulation. Hepatology. 2014;60:1196–210.
Friedman SL. Liver fibrosis—from bench to bedside. J Hepatol. 2003;38:S38–53.
Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73.
van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449–57.
Yu SJ, Kim W, Kim D, Yoon JH, Lee K, Kim JH, et al. Visceral obesity predicts significant fibrosis in patients with nonalcoholic fatty liver disease. Medicine. 2015;94:e2159.
Jun DW, Han JH, Kim SH, Jang EC, Kim NI, Lee JS, et al. Association between low thigh fat and non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2008;23:888–93.
Cao L, Quan XB, Zeng WJ, Yang XO, Wang MJ. Mechanism of hepatocyte apoptosis. J Cell Death. 2016;9:19–29.
Feldstein AE, Gores GJ. Apoptosis in alcoholic and nonalcoholic steatohepatitis. Front Biosci. 2005;10:3093–9.
Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–43.
Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191–6.
Yang GL, Jia LQ, Wu J, Ma YX, Cao HM, Song N, et al. Effect of tanshinone IIA on oxidative stress and apoptosis in a rat model of fatty liver. Exp Ther Med. 2017;14:4639–46.
Yu AS, Keeffe EB. Elevated AST or ALT to nonalcoholic fatty liver disease: accurate predictor of disease prevalence? Am J Gastroenterol. 2003;98:955–6.
Cui B, Liu S, Lin X, Wang J, Li S, Wang Q, et al. Effects of Lycium barbarum aqueous and ethanol extracts on high-fat-diet induced oxidative stress in rat liver tissue. Molecules. 2011;16:9116–28.
Ginter E, Bobek P, Jurcovicova M. Role of L-ascorbic acid in lipid metabolism. In: Seib PA, Tolbert BM, editors. Ascorbic acid: chemistry, metabolism and uses. Washington DC: American Chemical Society; 1982. p. 381–93.
Bordia AK. The effect of vitamin C on blood lipids, fibrinolytic activity and platelet adhesiveness in patients with coronary artery disease. Atherosclerosis. 1980;35:181–7.
Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas I, Papademetriou L, Economou M, et al. The implication of obesity on total antioxidant capacity in apparently healthy men and women: the ATTICA study. Nutr Metab Cardiovasc Dis. 2007;17:590–7.
Bahadoran Z, Golzarand M, Mirmiran P, Shiva N, Azizi F. Dietary total antioxidant capacity and the occurrence of metabolic syndrome and its components after a 3-year follow-up in adults: Tehran Lipid and Glucose Study. Nutr Metab. 2012;9:70.
Acknowledgements
This work supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MEST) (2015R1A1A3A04001016 and 2018R1D1A1B07042585) and the Korea Health Industry Development Institute (KHIDI) Grant funded by the Korea Government (MHW) (HI16C0753).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lee, H., Ahn, J., Shin, S.S. et al. Ascorbic acid inhibits visceral obesity and nonalcoholic fatty liver disease by activating peroxisome proliferator-activated receptor α in high-fat-diet-fed C57BL/6J mice. Int J Obes 43, 1620–1630 (2019). https://doi.org/10.1038/s41366-018-0212-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0212-0
This article is cited by
-
Vitamin C status and its change in relation to glucose-lipid metabolism in overweight and obesity patients following laparoscopic sleeve gastrectomy
European Journal of Clinical Nutrition (2022)
-
Association of hypertriglyceridemic waist phenotype with non-alcoholic fatty liver disease: a cross-sectional study in a Chinese population
Hormones (2022)
-
Comparative RNA-Seq transcriptome analyses reveal dynamic time-dependent effects of 56Fe, 16O, and 28Si irradiation on the induction of murine hepatocellular carcinoma
BMC Genomics (2020)
-
A New Treatment for Local Adiposity with Ascorbic Acid and Ascorbyl-Palmitate Solution: Clinical and Histological Study
Aesthetic Plastic Surgery (2020)
-
The anti-obesity effects of Tongbi-san in a high-fat diet-induced obese mouse model
BMC Complementary and Alternative Medicine (2019)