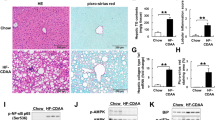
Abstract
Background/Aim
Nonalcoholic fatty liver disease (NAFLD) is characterized by hepatic steatosis, impaired insulin sensitivity, and chronic low-grade inflammation. Our previous studies indicated that zinc alpha2 glycoprotein (ZAG) alleviates palmitate (PA)-induced intracellular lipid accumulation in hepatocytes. This study is to further characterize the roles of ZAG on the development of hepatic steatosis, insulin resistance (IR), and inflammation.
Methods
ZAG protein levels in the livers of NAFLD patients, high-fat diet (HFD)-induced or genetically (ob/ob) induced obese mice, and in PA-treated hepatocytes were determined by western blotting. C57BL/6J mice injected with an adenovirus expressing ZAG were fed HFD for indicated time to induce hepatic steatosis, IR, and inflammation, and then biomedical, histological, and metabolic analyses were conducted to identify pathologic alterations in these mice. The molecular mechanisms underlying ZAG-regulated hepatic steatosis were further explored and verified in mice and hepatocytes.
Results
ZAG expression was decreased in NAFLD patient liver biopsy samples, obese mice livers, and PA-treated hepatocytes. Simultaneously, ZAG overexpression alleviated intracellular lipid accumulation via upregulating adiponectin and lipolytic genes (FXR, PPARα, etc.) while downregulating lipogenic genes (SREBP-1c, LXR, etc.) in obese mice as well as in cultured hepatocytes. ZAG improved insulin sensitivity and glucose tolerance via activation of IRS/AKT signaling. Moreover, ZAG significantly inhibited NF-ĸB/JNK signaling and thus resulting in suppression of obesity-associated inflammatory response in hepatocytes.
Conclusions
Our results revealed that ZAG could protect against NAFLD by ameliorating hepatic steatosis, IR, and inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bzowej NH. Nonalcoholic steatohepatitis: the new frontier for liver transplantation. Curr Opin Organ Transplant. 2018. https://doi.org/10.1097/MOT.0000000000000502.
Chen Z, Yu R, Xiong Y, Du F, Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16:203.
Polyzos SA, Kountouras J, Mantzoros CS. Adipokines in nonalcoholic fatty liver disease. Metabolism. 2016;65:1062–79.
Adolph TE, Grander C, Grabherr F, Tilg H, Adipokines and non-alcoholic fatty liver disease: multiple interactions. Int J Mol Sci. 2017;18:E1649
Ruan H, Dong LQ. Adiponectin signaling and function in insulin target tissues. J Mol Cell Biol. 2016;8:101–9.
Burgi W, Schmid K. Preparation and properties of Zn-alpha 2-glycoprotein of normal human plasma. J Biol Chem. 1961;236:1066–74.
Bing C, Bao Y, Jenkins J, Sanders P, Manieri M, Cinti S, et al. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci USA. 2004;101:2500–5.
Liao X, Wang X, Li H, Li L, Zhang G, Yang M, et al. Sodium-glucose cotransporter 2 (SGLT2) inhibitor increases circulating zinc-alpha2-glycoprotein levels in patients with type 2 diabetes. Sci Rep. 2016;6:32887.
Russell ST, Tisdale MJ. Role of beta-adrenergic receptors in the anti-obesity and anti-diabetic effects of zinc-alpha2-glycoprotein (ZAG). Biochim Biophys Acta. 2012;1821:590–9.
Xiao XH, Qi XY, Wang YD, Ran L, Yang J, Zhang HL, et al. Zinc alpha2 glycoprotein promotes browning in adipocytes. Biochem Biophys Res Commun. 2018. https://doi.org/10.1016/j.bbrc.2018.01.039.
Balaz M, Vician M, Janakova Z, Kurdiova T, Surova M, Imrich R, et al. Subcutaneous adipose tissue zinc-alpha2-glycoprotein is associated with adipose tissue and whole-body insulin sensitivity. Obesity (Silver Spring). 2014;22:1821–9.
Gao D, Trayhurn P, Bing C. Macrophage-secreted factors inhibit ZAG expression and secretion by human adipocytes. Mol Cell Endocrinol. 2010;325:135–42.
Xiao X, Li H, Qi X, Wang Y, Xu C, Liu G, et al. Zinc alpha2 glycoprotein alleviates palmitic acid-induced intracellular lipid accumulation in hepatocytes. Mol Cell Endocrinol. 2017;439:155–64.
Fan G, Qiao Y, Gao S, Guo J, Zhao R, Yang X. Effects of zinc alpha2 glycoprotein on lipid metabolism of liver in high-fat diet-induced obese mice. Horm Metab Res. 2017;49:793–800.
Cang X, Wang X, Liu P, Wu X, Yan J, Chen J, et al. PINK1 alleviates palmitate induced insulin resistance in HepG2 cells by suppressing ROS mediated MAPK pathways. Biochem Biophys Res Commun. 2016;478:431–8.
Younossi ZM, Loomba R, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, et al. Current and future therapeutic regimens for non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Hepatology. 2017. https://doi.org/10.1002/hep.29724.
Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2017. https://doi.org/10.1007/s00535-017-1415-1.
Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol. 2017;8:1–8.
Gan L, Xiang W, Xie B, Yu L. Molecular mechanisms of fatty liver in obesity. Front Med. 2015;9:275–87.
Montagner A, Polizzi A, Fouche E, Ducheix S, Lippi Y, Lasserre F, et al. Liver PPARalpha is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65:1202–14.
Tanaka N, Aoyama T, Kimura S, Gonzalez FJ. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol Ther. 2017;179:142–57.
Berlanga A, Guiu-Jurado E, Porras JA, Auguet T. Molecular pathways in non-alcoholic fatty liver disease. Clin Exp Gastroenterol. 2014;7:221–39.
Chen Q, Wang T, Li J, Wang S, Qiu F, Yu H, et al. Effects of natural products on fructose-induced nonalcoholic fatty liver disease (NAFLD). Nutrients. 2017;9. pii: E96. https://doi.org/10.3390/nu9020096.
Xiong X, Wang X, Lu Y, Wang E, Zhang Z, Yang J, et al. Hepatic steatosis exacerbated by endoplasmic reticulum stress-mediated downregulation of FXR in aging mice. J Hepatol. 2014;60:847–54.
Cui CX, Deng JN, Yan L, Liu YY, Fan JY, Mu HN, et al. Silibinin capsules improves high fat diet-induced nonalcoholic fatty liver disease in hamsters through modifying hepatic de novo lipogenesis and fatty acid oxidation. J Ethnopharmacol. 2017;208:24–35.
Zhan Z, Ren H, Peng ML. Role of CD36 in nonalcoholic fatty liver disease. Zhonghua Gan Zang Bing Za Zhi. 2017;25:953–6.
Liu M, Liu F. Up- and down-regulation of adiponectin expression and multimerization: mechanisms and therapeutic implication. Biochimie. 2012;94:2126–30.
Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–89.
Elattar S, Dimri M, Satyanarayana A. The tumor secretory factor ZAG promotes white adipose tissue browning and energy wasting. FASEB J. 2018. https://doi.org/10.1096/fj.201701465RR.
Dongiovanni P, Stender S, Pietrelli A, Mancina RM, Cespiati A, Petta S, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2017. https://doi.org/10.1111/joim.12719.
Geisler CE, Renquist BJ. Hepatic lipid accumulation: cause and consequence of dysregulated glucoregulatory hormones. J Endocrinol. 2017;234:R1–21.
Montgomery MK, Hallahan NL, Brown SH, Liu M, Mitchell TW, Cooney GJ, et al. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia. 2013;56:1129–39.
Samocha-Bonet D, Chisholm DJ, Tonks K, Campbell LV, Greenfield JR. Insulin-sensitive obesity in humans—a ‘favorable fat’ phenotype? Trends Endocrinol Metab. 2012;23:116–24.
Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol. 2014;220:T1–23.
Bhattacharyya S, Feferman L, Tobacman JK. Carrageenan inhibits insulin signaling through GRB10-mediated decrease in Tyr(P)-IRS1 and through inflammation-induced increase in Ser(P)307-IRS1. J Biol Chem. 2015;290:10764–74.
Geidl-Flueck B, Gerber PA. Insights into the hexose liver metabolism-glucose versus fructose. Nutrients. 2017;9. pii: E1026. https://doi.org/10.3390/nu9091026.
Ke B, Zhao Z, Ye X, Gao Z, Manganiello V, Wu B, et al. Inactivation of NF-kappaB p65 (RelA) in liver improves insulin sensitivity and inhibits cAMP/PKA pathway. Diabetes. 2015;64:3355–62.
Qu C, Zhou X, Yang G, Li L, Liu H, Liang Z. The natural logarithm of zinc-alpha2-glycoprotein/HOMA-IR is a better predictor of insulin sensitivity than the product of triglycerides and glucose and the other lipid ratios. Cytokine. 2016;79:96–102.
Xiang M, Wang PX, Wang AB, Zhang XJ, Zhang Y, Zhang P, et al. Targeting hepatic TRAF1-ASK1 signaling to improve inflammation, insulin resistance, and hepatic steatosis. J Hepatol. 2016;64:1365–77.
Xie L, Wang PX, Zhang P, Zhang XJ, Zhao GN, Wang A, et al. DKK3 expression in hepatocytes defines susceptibility to liver steatosis and obesity. J Hepatol. 2016;65:113–24.
Liu Y, Wang T, Liu X, Wei X, Xu T, Yin M, et al. Neuronal zinc-alpha2-glycoprotein is decreased in temporal lobe epilepsy in patients and rats. Neuroscience. 2017;357:56–66.
Leal VO,Lobo JC,Stockler-Pinto MB,Farage NE,Abdalla DS,Leite M,Jr. et al. Is zinc-alpha2-glycoprotein a cardiovascular protective factor for patients undergoing hemodialysis? Clin Chim Acta. 2012;413:616–9.
Welters ID, Bing C, Ding C, Leuwer M, Hall AM. Circulating anti-inflammatory adipokines high molecular weight adiponectin and zinc-alpha2-glycoprotein (ZAG) are inhibited in early sepsis, but increase with clinical recovery: a pilot study. BMC Anesthesiol. 2014;14:124.
Guo J, Liu Z, Sun H, Huang Y, Albrecht E, Zhao R. Lipopolysaccharide challenge significantly influences lipid metabolism and proteome of white adipose tissue in growing pigs. Lipids Health Dis. 2015;14:68.
Gao L, Wang PX, Zhang Y, Yu CJ, Ji Y, Wang X, et al. Tumor necrosis factor receptor-associated factor 5 (Traf5) acts as an essential negative regulator of hepatic steatosis. J Hepatol. 2016;65:125–36.
Zhu LH, Wang A, Luo P, Wang X, Jiang DS, Deng W, et al. Mindin/Spondin 2 inhibits hepatic steatosis, insulin resistance, and obesity via interaction with peroxisome proliferator-activated receptor alpha in mice. J Hepatol. 2014;60:1046–54.
An S, Zhao LP, Shen LJ, Wang S, Zhang K, Qi Y, et al. USP18 protects against hepatic steatosis and insulin resistance through its deubiquitinating activity. Hepatology. 2017;66:1866–84.
Liu M, Zhu H, Dai Y, Pan H, Li N, Wang L, et al. Zinc-alpha2-glycoprotein is associated with obesity in Chinese people and HFD-induced obese mice. Front Physiol. 2018;9:62.
Eckardt K, Schober A, Platzbecker B, Mracek T, Bing C, Trayhurn P, et al. The adipokine zinc-alpha2-glycoprotein activates AMP kinase in human primary skeletal muscle cells. Arch Physiol Biochem. 2011;117:88–93.
Sanders PM, Tisdale MJ. Effect of zinc-alpha2-glycoprotein (ZAG) on expression of uncoupling proteins in skeletal muscle and adipose tissue. Cancer Lett. 2004;212:71–81.
Herweijer H, Wolff JA. Gene therapy progress and prospects: hydrodynamic gene delivery. Gene Ther. 2007;14:99–107.
Siddiqui WH, Buttar HS. Pharmacokinetics of triclosan in rat after intravenous and intravaginal administration. J Environ Pathol Toxicol. 1979;2:861–71.
Lei L, Li K, Li L, Fang X, Zhou T, Zhang C, et al. Circulating zinc-α2-glycoprotein levels are low in newly diagnosed patients with metabolic syndrome and correlate with adiponectin. Nutr Metab (Lond). 2017;14:53.
Tian M, Liang Z, Liu R, Li K, Tan X, Luo Y, et al. Effects of sitagliptin on circulating zinc-α2-glycoprotein levels in newly diagnosed type 2 diabetes patients: a randomized trial. Eur J Endocrinol. 2016;174:147–55.
Xu L, Yu W, Niu M, Zheng C, Qu B, Li Y, et al. Serum ZAG levels were associated with eGFR mild decrease in T2DM patients with diabetic nephropathy. Int J Endocrinol. 2017;2017:5372625.
Lai Y, Chen J, Li L, Yin J, He J, Yang M, et al. Circulating zinc-α2-glycoprotein levels and insulin resistance in polycystic ovary syndrome. Sci Rep. 2016;6:25934.
Acknowledgments
This work was supported by research grants from the National Natural Science Foundation of China (81270925 and 81070667), Major Scientific Research Projects of Hunan Health and Family Planning Commission (A2017011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xiao, XH., Wang, YD., Qi, XY. et al. Zinc alpha2 glycoprotein protects against obesity-induced hepatic steatosis. Int J Obes 42, 1418–1430 (2018). https://doi.org/10.1038/s41366-018-0151-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0151-9
This article is cited by
-
Extensive Mendelian randomization study identifies potential causal risk factors for severe COVID-19
Communications Medicine (2021)
-
Serum zinc-α2-glycoprotein levels are elevated and correlated with thyroid hormone in newly diagnosed hyperthyroidism
BMC Endocrine Disorders (2019)