Abstract
Background/objectives
Spendthrift vs. thrifty individuals expend more energy and experience greater weight loss during caloric restriction (CR). Adaptive mechanisms in skeletal muscle, adipose tissue, and on hormone level modulate energy expenditure (EE) during weight loss. Metabolic mechanisms underlying the variability in EE during CR are unclear. The present study explored whether during long-term CR (i) gene expression changes in skeletal muscle and adipose tissue relate with the individual EE response and weight loss, and (ii) altered catecholamine and FGF21-concentrations are associated with measures of metabolic adaptation.
Subjects/methods
In a 10-week inpatient study, 24-h EE was measured before and after 6 weeks of 50% CR in 12 subjects using whole-room indirect calorimetry. Weight loss was assessed and repeated hormone measurements performed. Muscle and adipose tissue biopsies were taken before and after CR, and gene expression was assessed (RNA-Seq). Genes showing the most significant changes after CR were tested for association with EE and followed-up for further association with metabolic measures in a separate phenotyping study (n = 103).
Results
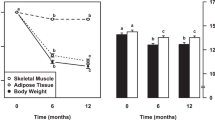
Muscle UCP2 showed the strongest change after CR (log2-fold change = −1.57, false discovery rate = 0.10) and was considered the best gene for exploration of metabolic adaptive processes. A greater decrease in UCP2-expression was associated with less weight loss (P = 0.03, r = 0.77) and relatively lower 24-h EE after CR (P = 0.001, r = −0.96). Post-CR changes in FGF21-plasma concentrations correlated with UCP2-expression change (P = 0.02, r = −0.89) and weight loss (P = 0.003, r = −0.83). In a separate metabolic phenotyping study, muscle UCP2-expression correlated with respiratory quotient and macronutrient oxidation. In adipose tissue, no candidate genes for metabolic exploration were found.
Conclusions
Changes in muscle UCP2-expression reflect an inter-individual metabolic response to long-term CR and may influence EE and weight loss via modulation of substrate oxidation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schlögl M, Piaggi P, Pannacciuli N, Bonfiglio SM, Krakoff J, Thearle MS. Energy expenditure responses to fasting and overfeeding identify phenotypes associated with weight change. Diabetes. 2015;64:3680–9.
Reinhardt M, Thearle MS, Ibrahim M, Hohenadel MG, Bogardus C, Krakoff J, et al. A human thrifty phenotype associated with less weight loss during caloric restriction. Diabetes. 2015;64:2859–67.
Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods results using a respiratory chamber. J Clin Investig. 1986;78:1568.
Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. New Engl J Med. 1995;332:621–8.
Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391.
Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau J-A, Heymsfield S, Joanisse DR, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol-Regul, Integr Comp Physiol. 2003;285:R183–92.
Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–86.
Baldwin KM, Joanisse DR, Haddad F, Goldsmith RL, Gallagher D, Pavlovich KH, et al. Effects of weight loss and leptin on skeletal muscle in human subjects. Am J Physiol-Regul, Integr Comp Physiol. 2011;301:R1259–66.
Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–25.
Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, et al. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci. 2009;106:10853–8.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. Series B (Methodological). 1995;57:289–300.
Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr. 1991;53:1368–71.
Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–12.
Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010;1:212–28.
Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J. Extent and determinants of thermogenic responses to 24 h of fasting, energy balance, and five different overfeeding diets in humans. J Clin Endocrinol Metab. 2013;98:2791–9.
Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes. 2013;62:4043–51.
Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab. 2013;98:E703–707.
Diano S, Horvath TL. Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol Med. 2012;18:52–8.
Kukat A, Dogan SA, Edgar D, Mourier A, Jacoby C, Maiti P, et al. Loss of UCP2 attenuates mitochondrial dysfunction without altering ROS production and uncoupling activity. PLoS Genet. 2014;10:e1004385.
Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, Bouillaud F, et al. Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J. 2008;22:9–18.
Walder K, Norman RA, Hanson RL, Schrauwen P, Neverova M, Jenkinson CP, et al. Association between uncoupling protein polymorphisms (UCP2–UCP3) and energy metabolism/obesity in Pima Indians. Hum Mol Genet. 1998;7:1431–5.
Klingenberg M. Mechanism and evolution of the uncoupling protein of brown adipose tissue. Trends Biochem Sci. 1990;15:108–12.
Dalgaard L, Pedersen O. Uncoupling proteins: functional characteristics and role in the pathogenesis of obesity and Type II diabetes. Diabetologia. 2001;44:946–65.
Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev. 1984;64:1–64.
Jia JJ, Zhang X, Ge CR, Jois M. The polymorphisms of UCP2 and UCP3 genes associated with fat metabolism, obesity and diabetes. Obes Rev. 2009;10:519–26.
Brand M, Brindle K, Buckingham J, Harper J, Rolfe D, Smart J. The significance and mechanism of mitochondrial proton conductance. Int J Obes. 1999;23:S4–11.
Rolfe DF, Newman JM, Buckingham JA, Clark MG, Brand MD. Contribution of mitochondrial proton leak to respiration rate in working skeletal muscle and liver and to SMR. Am J Physiol-Cell Physiol. 1999;276:C692–99.
Samec S, Seydoux J, Dulloo AG. Role of UCP homologues in skeletal muscles and brown adipose tissue: mediators of thermogenesis or regulators of lipids as fuel substrate? FASEB J. 1998;12:715–24.
Müller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, et al. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. Am J Clin Nutr. 2015;102:807–19.
Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007;86:625–32.
Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba B, Raz I, Saad M, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol-Endocrinol Metab. 1990;259:E650–57.
Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher daily energy expenditure and respiratory quotient, rather than fat-free mass, independently determine greater ad libitum overeating. J Clin Endocrinol Metab. 2015;100:3011–20.
Schrauwen P, Xia J, Bogardus C, Pratley RE, Ravussin E. Skeletal muscle uncoupling protein 3 expression is a determinant of energy expenditure in Pima Indians. Diabetes. 1999;48:146–9.
Jensen MD, Ekberg K, Landau BR. Lipid metabolism during fasting. Am J Physiol-Endocrinol Metab. 2001;281:E789–93.
Holmes D. Metabolism: Fasting induces FGF21 in humans. Nat Rev Endocrinol. 2016;12:3–3.
Fazeli PK, Lun M, Kim SM, Bredella MA, Wright S, Zhang Y, et al. FGF21 and the late adaptive response to starvation in humans. J Clin Invest. 2015;125:4601.
Uebanso T, Taketani Y, Yamamoto H, Amo K, Ominami H, Arai H, et al. Paradoxical regulation of human FGF21 by both fasting and feeding signals: is FGF21 a nutritional adaptation factor? PLoS ONE. 2011;6:e22976.
Weyer C, Snitker S, Rising R, Bogardus C, Ravussin E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. Int J Obes Relat Metab Disord. 1999;23:715–22.
Acknowledgements
We thank all volunteers who participated in this study.
Funding
National Institutes of Health Intramural Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Clinical Trial Registration: NCT00687115, NCT00340132; clinicaltrials.gov
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Heinitz, S., Piaggi, P., Yang, S. et al. Response of skeletal muscle UCP2-expression during metabolic adaptation to caloric restriction. Int J Obes 42, 974–984 (2018). https://doi.org/10.1038/s41366-018-0085-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0085-2
This article is cited by
-
Glycyrrhizin Improves Fatty Liver Symptoms, Increases Adiponectin and Reduces UCP2 Expression in Wistar Rats
Proceedings of the National Academy of Sciences, India Section B: Biological Sciences (2020)