Abstract
Background
Obesity contributes significantly to the development and evolution of cardiovascular disease (CVD) which is believed to be mediated by oxidative stress, inflammation and endothelial dysfunction. However, the vascular health of metabolically obese and normal weight (MONW) individuals is not completely comprehended.
Objectives
The purpose of our study was to evaluate vascular function on the basis of a high fat diet (HFD)-MONW rabbit model.
Subjects
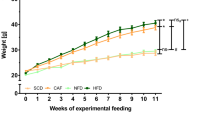
Twenty four male rabbits were randomly assigned to receive either a regular diet (CD, n = 12) or a high-fat diet (18% extra fat on the regular diet, HFD, n = 12) for 6 weeks.
Results
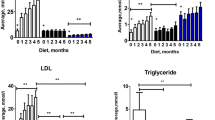
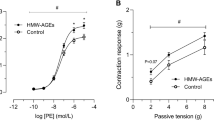
Body weight, TBARS and gluthathione serum levels were similar between the groups; fasting glucose, triglycerides, C reactive protein (CRP), visceral adipose tissue (VAT), triglyceride-glucose index (TyG index) were higher in the HFD group. Compared to CD, the HFD rabbits had glucose intolerance and lower HDL-cholesterol and plasma nitrites levels. Thoracic aortic rings from HFD rabbits exhibited: (a) a reduced acetylcholine-induced vasorelaxation; (b) a greater contractile response to norepinephrine and KCl; (c) an improved angiotensin II-sensibility. The HFD-effect on acetylcholine-response was reversed by the cyclooxygenase-2 (COX-2) inhibitor (NS398) and the cyclooxygenase-1 inhibitor (SC560), and the HFD-effect on angiotensin II was reversed by NS398 and the TP receptor blocker (SQ29538). Immunohistochemistry and western blot studies showed COX-2 expression only in arteries from HFD rabbits.
Conclusions
Our study shows a positive pro-inflammatory status of HFD-induced MONW characterized by raised COX-2 expression, increase of the CRP levels, reduction of NO release and oxidative stress-controlled conditions in an early stage of metabolic alterations characteristic of metabolic syndrome. Endothelial dysfunction and increased vascular reactivity in MONW individuals may be biomarkers of early vascular injury. Therefore, the metabolic changes induced by HFD even in normal weight individuals may be associated to functional alterations of blood vessels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26:968–76.
Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case–control study. Lancet. 2005;366:1640–9.
WHO Expert Committee on Physical Status: the use and interpretation of anthropometry. Physical status: the use and interpretation of anthropometry 1995. Geneva, Switzerland: World Health Organization; 1993.
Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96.
Frankenfield DC, Rowe WA, Cooney RN, Smiths JS, Becker D. Limits of body mass index to detect obesity and predict body composition. Nutrition. 2001;17:26–30.
Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32:959–66.
Dobson R, Burgess MI, Sprung VS, Irwin A, Hamer M, Jones J, et al. Metabolically healthy and unhealthy obesity: differential effects on myocardial function according to metabolic syndrome, rather than obesity. Int J Obes. 2016;40:153–61.
Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713.
Wang B, Zhuang R, Luo X, Yin L, Pang C, Feng T, et al. Prevalence of metabolically healthy obese and metabolically obese but normal weight in adults worldwide: a meta-analysis. Horm Metab Res. 2015;47:839–45.
Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31:737–46.
Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–2600.
Esposito K, Giugliano D. The metabolic syndrome and inflammation: association or causation? Nutr Metab Cardiovasc Dis. 2004;14:228–32.
Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Endothelial dysfunction in metabolic syndrome: prevalence, pathogenesis and management. Nutr Metab Cardiovasc Dis. 2010;20:140–6.
Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metab Clin Exp. 2006;55:928–34.
Busija DW, Miller AW, Katakam P, Erdos B. Adverse effects of reactive oxygen species on vascular reactivity in insulin resistance. Antioxid Redox Signal. 2006;8:1131–40.
Carlström M, Larsen FJ, Nyström T, Hezel M, Borniquel S, Weitzberg E, et al. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc Natl Acad Sci USA. 2010;107:17716–20.
Huang K, Huang Y, Frankel J, Addis C, Jaswani L, Wehner PS, et al. The short-term consumption of a moderately high-fat diet alters nitric oxide bioavailability in lean female Zucker rats. Can J Physiol Pharmacol. 2011;89:245–57.
Litvinova L, Atochin DN, Fattakhov N, Vasilenko M, Zatolokin P, Kirienkova E. Nitric oxide and mitochondria in metabolic syndrome. Front Physiol. 2015;6:20.
Vessières E, Belin de Chantemèle EJ, Guihot AL, Jardel A, Loufrani L, Henrion D. Cyclooxygenase-2 inhibition restored endothelium-mediated relaxation in old obese Zucker rat mesenteric arteries. Front Physiol. 2010;1:145.
Tian XY, Wong WT, Leung FP, Zhang Y, Wang YX, Lee HK, et al. Oxidative stress-dependent cyclooxygenase-2-derived prostaglandin F2a impairs endothelial function in renovascular hypertensive rats. Antioxid Redox Signal. 2012;16:363–73.
Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2008;294:H1658–66.
Xiang L, Naik JS, Hodnett BL, Hester RL. Altered arachidonic acid metabolism impairs functional vasodilation in metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2006;90:R134–8.
Alarcon G, Roco J, Medina A, Van Nieuwenhoven C, Medina M, Jerez S. Stearoyl-CoA desaturase indexes and n-6/n-3 fatty acids ratio as biomarkers of cardiometabolic risk factors in normal-weight rabbits fed high fat diets. J Biom Sci. 2016;23:13.
Georgiev IP, Kanelov IN, Dimitrova SS, Iliev YI, Tanev SI, Georgieva TM, et al. An experimental model for evaluation of glucose tolerance in rabbit. Bulg J Vet Med. 2006;9:27–35.
Lee S-H, Han K, Yang HK, Kim HS, Cho JH, Kwon HS, et al. A novel criterion for identifying metabolically obese but normal weight individuals using the product of triglycerides and glucose. Nutr Diab. 2015;5:e149.
Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comp euglycemic-hyperinsulinemic clamp J Clin Endocrinol Metab. 2010;95:3347–51.
Moshage H, Kok B, Huizenga JR, Jansen PLM. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem. 1995;6:892–6.
Karbiner MS, Sierra L, Minahk C, Fonio MC, Peral de Bruno M, Jerez S. The role of oxidative stress in alterations of hematological parameters and inflammatory markers induced by early hypercholesterolemia. Life Sci. 2013;93:503–8.
Cao L, Liu X, Cao H, Lv Q, Tong N. Modified high-sucrose diet-induced abdominally obese and normal-weight rats developed high plasma free fatty acid and insulin resistance. Oxid Med Cell Longev. 2012;2012:374346.
Stapleton PA, James ME, Goodwill AG, Frisbee JC. Obesity and vascular dysfunction. Pathophysiol. 2008;15:79–89.
Singhal A. Endothelial dysfunction: role in obesity-related disorders and the early origins of CVD. Proc Nutr Soc. 2005;64:15–22.
Yudkin JS. Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes. 2003;27:S25–28.
Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, Popma JJ, Stevenson W. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation. 2006;114:2850–70.
Jerez S, Scacchi F, Sierra L, Karbiner S, Peral de Bruno M. Vascular hyporeactivity to angiotensin ii and noradrenaline in a rabbit model of obesity. J Cardiovasc Pharmacol. 2012;59:49–57.
Vaccaro O, Imperatore G, IovinoV, Iovine C, Rivellese A, Riccardi G. Does impaired glucose tolerance predict hypertension? Diabetologia. 1996;39:70–76.
Siddiqui A, Hussain T. Enhanced AT1 receptor mediated vasocontractile response to angiotensin II in endothelium denuded aorta of obese Zucker rat. Am J Physiol. 2006;292:H1722–27.
Bhattacharya I, Mundy AL, Widmer CC, Kretz M, Barton M. Regional heterogeneity of functional changes in conduit arteries after high-fat diet. Obesity . 2008;16:743–8.
Thorp AA, Schlaich MP. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J Diabetes Res. 2015;2015:341583.
Sandoo A, van Zanten JJCV, Metsios GS, Carroll D, Kitas GD. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 2010;4:302–12.
Jerez S, Peral de Bruno M, Coviello A. Nitric oxide modulates angiotensin II-induced endothelial vasoconstrictor prostanoid reléase. Eur J Pharmacol. 2005;520:127–34.
Medina M, Alberto MR, Sierra L, Van Nieuwenhove C, Saad S, Isla MI, Jerez S. Hypercholesterolemia increases plasma saturated and n-6 fatty acids altering prostaglandin homeostasis and promotes endothelial dysfunction in rabbits. Lipids. 2014;49:685–93.
Ridker P, Morrow DA. C-reactive protein, inflammation, and coronary risk. Cardiol Clin. 2003;21:313–25.
Lee JY, Hwang DH. The modulation of inflammatory gene expression by lipids: mediation through Toll-like receptors. Mol Cells. 2006;21:174–85.
Hellmann J, Zhang MJ, Tang Y, Rane M, Bhatnagar A, Spite M. Increased saturated fatty acids in obesity alter resolution of inflammation in part by stimulating prostaglandin production. J Immunol. 2013;191:1383–92.
Hwang D. Modulation of the expression of cyclooxygenase-2 by fatty acids mediated through Toll-like receptor 4-derived signaling pathways. FASEB J. 2001;15:2556–64.
Toborek M, Lee YW, Garrido R, Kaiser S, Hennig B. Unsaturated fatty acids selectively induce an inflammatory environment in human endothelial cells. Am J Clin Nutr. 2002;75:119–25.
Hennig B, Lei W, Arzuaga X, Ghosh DD, Saraswathi V, Toborek M. Linoleic acid induces proinflammatory events in vascular endothelial cells via activation of PI3K/Akt and ERK1/2 signaling. J Nutr Biochem. 2006;17:766–72.
Morteau O. Prostaglandins and inflammation: the cyclooxygenase controversy. Arch Immunol Ther Exp. 2000;48:473–80.
Acknowledgements
This work was supported by grants from the Consejo de Investigaciones de la Universidad Nacional de Tucumán (PIUNT I521/1), Consejo de Investigaciones Científicas y Técnicas de la República Argentina (CONICET PIP 215/14), Universidad del Norte Santo Tomás de Aquino and Institutional funds from INSIBIO (Instituto Superior de Investigaciones Biológicas). We thank veterinary Rosa Alejandra Molina for bioterio management and Dr Liliana Sierra for her help in data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Alarcon, G., Roco, J., Medina, M. et al. High fat diet-induced metabolically obese and normal weight rabbit model shows early vascular dysfunction: mechanisms involved. Int J Obes 42, 1535–1543 (2018). https://doi.org/10.1038/s41366-018-0020-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0020-6
This article is cited by
-
Pathophysiological adaptations of resistance arteries in rat offspring exposed in utero to maternal obesity is associated with sex-specific epigenetic alterations
International Journal of Obesity (2021)
-
The association between olfactory dysfunction and cardiovascular disease and its risk factors in middle-aged and older adults
Scientific Reports (2021)
-
7,8-Dihydroxyflavone alleviated the high-fat diet and alcohol-induced memory impairment: behavioral, biochemical and molecular evidence
Psychopharmacology (2020)