Abstract
Background
Impaired sympathetic/parasympathetic response, expressed by elevated Acetylcholinesterase (AChE) is associated with obesity, metabolic syndrome and inflammation. However, the association between morbid obesity and AChE and the changes in cholinergic tone following bariatric laparoscopic sleeve gastrectomy (LSG) surgery-induced weight reduction were never analyzed.
Methods
Two studies are presented; the first (the “apparently healthy cohort”) was a cross-sectional study and the second (the “LSG cohort”) was a prospective-cohort study with 12 months of follow-up. The “apparently healthy cohort” included 1450 apparently healthy participants who volunteered to the Tel-Aviv Medical Center Inflammation Survey (TAMCIS) during a routine annual checkup visit. The “LSG cohort” included 77 morbid obese patients before and at 3, 6, and 12 months following LSG surgery. Main outcomes included anthropometric measurements, Hemoglobin A1c (HbA1C), serum AChE, insulin test and Homeostasis Model Assessment (HOMA).
Results
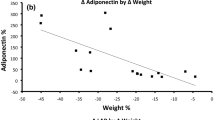
Among the TAMCIS participants, serum AChE activity increased with BMI in a dose-dependent manner until it reached a peak level at BMI of 30–35 kg/m², followed by a plateau. Following LSG, a significant decrease in AChE activity between baseline and 12 months post-surgery was found for men, but not for women (−122.2 ± 135.3, P < 0.001 vs. −21.8 ± 120.5, P = 0.258 nmol substrate hydrolyzed/min per ml, respectively). The reduction in AChE activity was negatively correlated with %excess weight loss (EWL) and positively correlated with %body fat reduction at 12 months post-surgery among women (r = −0.329, P = 0.034 and r = 0.350, P = 0.023, respectively). In men, AChE activity reduction was positively correlated with the HOMA reduction (r = 0.358, P = 0.048).
Conclusions
Obesity-related AChE resistance phenotype may be reversed following LSG and correlates with metabolic outcomes. Further long-term studies will be needed to validate and evaluate the beneficial effect of AChE reduction post bariatric surgery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gonzalez-Muniesa P, et al. Obesity. Nat Rev Dis Prim. 2017;3:17034.
Runkel N, et al. Bariatric surgery. Dtsch Arzteblatt Int. 2011;108:341–6.
de Lima KV, Costa MJ, Goncalves Mda C, Sousa BS. Micronutrient deficiencies in the pre-bariatric surgery. Arq Bras De Cir Dig. 2013;26:63–6.
Benaiges D, et al. Laparoscopic sleeve gastrectomy: more than a restrictive bariatric surgery procedure? World J Gastroenterol. 2015;21:11804–14.
Sweeney TE, Morton JM. Metabolic surgery: action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Pract Res Clin Gastroenterol. 2014;28:727–40.
Magouliotis DE, Tasiopoulou VS, Sioka E, Chatedaki C, Zacharoulis D. Impact of bariatric surgery on metabolic and gut microbiota profile: a systematic review and meta-analysis. Obes Surg. 2017;27:1345–57.
Tentolouris N, Liatis S, Katsilambros N. Sympathetic system activity in obesity and metabolic syndrome. Ann N Y Acad Sci. 2006;1083:129–52.
Poirier P, Hernandez TL, Weil KM, Shepard TJ, Eckel RH. Impact of diet-induced weight loss on the cardiac autonomic nervous system in severe obesity. Obes Res. 2003;11:1040–7.
Karason K, Molgaard H, Wikstrand J, Sjostrom L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83:1242–7.
Ofek K, Soreq H. Cholinergic involvement and manipulation approaches in multiple system disorders. Chem-Biol Interact. 2013;203:113–9.
Shenhar-Tsarfaty S, Berliner S, Bornstein NM, Soreq H. Cholinesterases as biomarkers for parasympathetic dysfunction and inflammation-related disease. J Mol Neurosci. 2014;53:298–305.
Myers MG Jr, Olson DP. Central nervous system control of metabolism. Nature. 2012;491:357–63.
Thorp AA, Schlaich MP. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J Diabetes Res. 2015;2015:341583.
Metz CN, Tracey KJ. It takes nerve to dampen inflammation. Nat Immunol. 2005;6:756–57.
Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62.
Soreq H, Seidman S. Acetylcholinesterase--new roles for an old actor. Nat Rev Neurosci. 2001;2:294–302.
Das UN. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med Sci Monit. 2007;13:Ra214–21.
Kakinuma Y, et al. Acetylcholine from vagal stimulation protects cardiomyocytes against ischemia and hypoxia involving additive non-hypoxic induction of HIF-1alpha. FEBS Lett. 2005;579:2111–8.
Ando M, et al. Efferent vagal nerve stimulation protects heart against ischemia-induced arrhythmias by preserving connexin43 protein. Circulation. 2005;112:164–70.
Dewland TA, Androne AS, Lee FA, Lampert RJ, Katz SD. Effect of acetylcholinesterase inhibition with pyridostigmine on cardiac parasympathetic function in sedentary adults and trained athletes. Am J Physiol Heart Circ Physiol. 2007;293:H86–92.
Androne AS, Hryniewicz K, Goldsmith R, Arwady A, Katz SD. Acetylcholinesterase inhibition with pyridostigmine improves heart rate recovery after maximal exercise in patients with chronic heart failure. Heart. 2003;89:854–8.
Canaani J, et al. Serum ache activities predict exercise heart rate parameters of asymptomatic individuals. Neurosci Med. 2010;1:43–9.
Meydan C, Shenhar-Tsarfaty S, Soreq H. MicroRNA regulators of anxiety and metabolic disorders. Trends Mol Med. 2016;22:798–812.
Shenhar-Tsarfaty S, et al. Weakened cholinergic blockade of inflammation associates with diabetes-related depression. Mol Med. 2016;22:156–161.
Arbel Y, et al. Decline in serum cholinesterase activities predicts 2-year major adverse cardiac events. Mol Med. 2014;20:38–45.
Ben Assayag E, et al. Serum cholinesterase activities distinguish between stroke patients and controls and predict 12-month mortality. Mol Med. 2010;16:278–86.
Sherf-Dagan S, et al. Probiotics administration following sleeve gastrectomy surgery: a randomized double-blind trial. Int J Obes. 2017.
Sherf Dagan S, et al. Inadequate protein intake after laparoscopic sleeve gastrectomy surgery is associated with a greater fat free mass loss. Surg Obes Relat Dis. 2017;13:101–9.
Sherf Dagan S, et al. Do bariatric patients follow dietary and lifestyle recommendations during the first postoperative year? Obes Surg. 2017;27:2258–71.
Toolabi K, Arefanian S, Golzarand M, Arefanian H. Effects of laparoscopic Roux-en-Y gastric bypass (LRYGB) on weight loss and biomarker parameters in morbidly obese patients: a 12-month follow-up. Obes Surg. 2011;21:1834–42.
Mirrakhimov AE, et al. Cut off values for abdominal obesity as a criterion of metabolic syndrome in an ethnic Kyrgyz population (Central Asian region). Cardiovasc Diabetol. 2012;11:16.
Ellman GL, Courtney KD, Andres V Jr., Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95.
Shenhar-Tsarfaty S, et al. Fear and C-reactive protein cosynergize annual pulse increases in healthy adults. Proc Natl Acad Sci USA. 2015;112:E467–71.
Birikh KR, Sklan EH, Shoham S, Soreq H. Interaction of “readthrough” acetylcholinesterase with RACK1 and PKCbeta II correlates with intensified fear-induced conflict behavior. Proc Natl Acad Sci USA. 2003;100:283–8.
Cruz KJ, de Oliveira AR, Morais JB, Severo JS, Marreiro PhDD. Role of microRNAs on adipogenesis, chronic low-grade inflammation, and insulin resistance in obesity. Nutrition. 2017;35:28–35.
Straznicky NE, Eikelis N, Lambert EA, Esler MD. Mediators of sympathetic activation in metabolic syndrome obesity. Curr Hypertens Rep. 2008;10:440–7.
Grassi G, et al. Regional differences in sympathetic activation in lean and obese normotensive individuals with obstructive sleep apnoea. J Hypertens. 2014;32:383–8.
Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162.
Sklan EH, et al. Acetylcholinesterase/paraoxonase genotype and expression predict anxiety scores in Health, Risk Factors, Exercise Training, and Genetics study. Proc Natl Acad Sci USA. 2004;101:5512–7.
Young MT, Phelan MJ, Nguyen NT. A decade analysis of trends and outcomes of male vs female patients who underwent bariatric surgery. J Am Coll Surg. 2016;222:226–31.
Farinholt GN, Carr AD, Chang EJ, Ali MR. A call to arms: obese men with more severe comorbid disease and underutilization of bariatric operations. Surg Endosc. 2013;27:4556–63.
Stefanidis A, Oldfield BJ. Neuroendocrine mechanisms underlying bariatric surgery: Insights from human studies and animal models. J Neuroendocrinol. 2017;29.
Browning KN, Fortna SR, Hajnal A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J Physiol. 2013;591:2357–72.
Robinson AH, et al. What variables are associated with successful weight loss outcomes for bariatric surgery after 1 year? Surg Obes Relat Dis. 2014;10:697–704.
Wimmelmann CL, Dela F, Mortensen EL. Psychological predictors of weight loss after bariatric surgery: a review of the recent research. Obes Res Clin Pract. 2014;8:e299–313.
Ruiz-Lozano T, et al. Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clin Nutr. 2016.
Johnson Stoklossa C, Atwal S. Nutrition care for patients with weight regain after bariatric surgery. Gastroenterol Res Pract. 2013;2013:256145.
McGrice M, Don Paul K. Interventions to improve long-term weight loss in patients following bariatric surgery: challenges and solutions. Diabetes Metab Syndr Obes. 2015;8:263–74.
Miras AD, et al. Psychological characteristics, eating behavior, and quality of life assessment of obese patients undergoing weight loss interventions. Scand J Surg. 2015;104:10–7.
Lauti M, Kularatna M, Hill AG, MacCormick AD. Weight regain following sleeve gastrectomy-a systematic review. Obes Surg. 2016;26:1326–34.
Sheets CS, et al. Post-operative psychosocial predictors of outcome in bariatric surgery. Obes Surg. 2015;25:330–45.
Heber D, et al. Endocrine and nutritional management of the post-bariatric surgery patient: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4823–43.
Hood MM, et al. Managing severe obesity: understanding and improving treatment adherence in bariatric surgery. J Behav Med. 2016;39:1092–103.
Thibault R, Pichard C. Overview on nutritional issues in bariatric surgery. Curr Opin Clin Nutr Metab care. 2016;19:484–90.
Jones L, Cleator J, Yorke J. Maintaining weight loss after bariatric surgery: when the spectator role is no longer enough. Clin Obes. 2016;6:249–58.
Funding
This study was supported (in part) by grant no. 3–10470 from the Chief Scientist Office of the Ministry of Health, Israel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were approved by the institutional research committees in both participating hospitals and in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The LSG study was pre-registered in the NIH registration website (TRIAL no. NCT01922830).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Shenhar-Tsarfaty, S., Sherf-Dagan, S., Berman, G. et al. Obesity-related acetylcholinesterase elevation is reversed following laparoscopic sleeve gastrectomy. Int J Obes 43, 297–305 (2019). https://doi.org/10.1038/s41366-018-0014-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0014-4