Abstract
Background/objectives
Ghrelin, a stomach-derived hormone implicated in numerous behaviors including feeding, reward, stress, and addictive behaviors, acts by binding to the growth hormone secretagogue receptor (GHSR). Here, we present the development, verification, and initial characterization of a novel GHSR knockout (KO) Wistar rat model created with CRISPR genome editing.
Methods
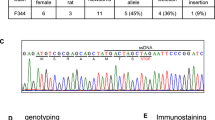
Using CRISPR/Cas9, we developed a GHSR KO in a Wistar background. Loss of GHSR mRNA expression was histologically verified using RNAscope in wild-type (WT; n = 2) and KO (n = 2) rats. We tested the effects of intraperitoneal acyl-ghrelin administration on food consumption and plasma growth hormone (GH) concentrations in WT (n = 8) and KO (n = 8) rats. We also analyzed locomotion, food consumption, and body fat composition in these animals. Body weight was monitored from early development to adulthood.
Results
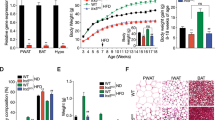
The RNAscope analysis revealed an abundance of GHSR mRNA expression in the hypothalamus, midbrain, and hippocampus in WTs, and no observed probe binding in KOs. Ghrelin administration increased plasma GH levels (p = 0.0067) and food consumption (p = 0.0448) in WT rats but not KOs. KO rats consumed less food overall at basal conditions and weighed significantly less compared with WTs throughout development (p = 0.0001). Compared with WTs, KOs presented higher concentrations of brown adipose tissue (BAT; p = 0.0322).
Conclusions
We have verified GHSR deletion in our KO model using histological, physiological, neuroendocrinological, and behavioral measures. Our findings indicate that GHSR deletion in rats is not only associated with a lack of response to ghrelin, but also associated with decreases in daily food consumption and body growth, and increases in BAT. This GHSR KO Wistar rat model provides a novel tool for studying the role of the ghrelin system in obesity and in a wide range of medical and neuropsychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660.
Gutierrez JA, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320–6325.
Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396.
Howard AD, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977.
Muller TD, et al. Ghrelin. Mol Metab. 2015;4:437–460.
Wren AM, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328.
Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913.
Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab. 2004;287:E297–E304.
Currie PJ, Mirza A, Fuld R, Park D, Vasselli JR. Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. Am J Physiol Regul Integr Comp Physiol. 2005;289:R353–R358.
Schele E, Bake T, Rabasa C, Dickson SL. Centrally administered ghrelin acutely influences food choice in rodents. PLoS ONE. 2016;11:e0149456.
Cummings DE, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719.
Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147:23–30.
Castaneda TR, Tong J, Datta R, Culler M, Tschop MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31:44–60.
Howick K, Griffin BT, Cryan JF, Schellekens H. From belly to brain: targeting the ghrelin receptor in appetite and food intake regulation. Int J Mol Sci. 2017;18:2.
Petersenn S, Rasch AC, Penshorn M, Beil FU, Schulte HM. Genomic structure and transcriptional regulation of the human growth hormone secretagogue receptor. Endocrinology. 2001;142:2649–2659.
Chow KB, et al. The truncated ghrelin receptor polypeptide (GHS-R1b) is localized in the endoplasmic reticulum where it forms heterodimers with ghrelin receptors (GHS-R1a) to attenuate their cell surface expression. Mol Cell Endocrinol. 2012;348:247–254.
Navarro G, et al. A significant role of the truncated ghrelin receptor GHS-R1b in ghrelin-induced signaling in neurons. J Biol Chem. 2016;291:13048–13062.
Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548.
Melis MR, et al. Ghrelin injected into the paraventricular nucleus of the hypothalamus of male rats induces feeding but not penile erection. Neurosci Lett. 2002;329:339–343.
Papotti M, et al. Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab. 2000;85:3803–3807.
Jerlhag E, et al. Requirement of central ghrelin signaling for alcohol reward. PNAS Proc Natl Acad Sci USA. 2009;106:11318–11323.
Zhao TJ, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107:7467–7472.
Albarran-Zeckler RG, Sun Y, Smith RG. Physiological roles revealed by ghrelin and ghrelin receptor deficient mice. Peptides. 2011;32:2229–2235.
Kang K, Zmuda E, Sleeman MW. Physiological role of ghrelin as revealed by the ghrelin and GOAT knockout mice. Peptides. 2011;32:2236–2241.
Sclafani A, Touzani K, Ackroff K. Ghrelin signaling is not essential for sugar or fat conditioned flavor preferences in mice. Physiol Behav. 2015;149:14–22.
Kouno T, et al. Reduced intake of carbohydrate prevents the development of obesity and impaired glucose metabolism in ghrelin O-acyltransferase knockout mice. Peptides. 2016;86:145–152.
Mear Y, Enjalbert A, Thirion S. GHS-R1a constitutive activity and its physiological relevance. Front Neurosci. 2013;7:87.
Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor--identification of a potent inverse agonist. Mol Endocrinol. 2003;17:2201–2210.
Bulbul M, et al. Food intake and interdigestive gastrointestinal motility in ghrelin receptor mutant rats. J Gastroenterol. 2011;46:469–478.
MacKay H, et al. Rats with a truncated ghrelin receptor (GHSR) do not respond to ghrelin, and show reduced intake of palatable, high-calorie food. Physiol Behav. 2016;163:88–96.
Clifford PS, et al. Attenuation of cocaine-induced locomotor sensitization in rats sustaining genetic or pharmacologic antagonism of ghrelin receptors. Addict Biol. 2012;17:956–963.
Clifford S, et al. Impact of food restriction and cocaine on locomotion in ghrelin- and ghrelin-receptor knockout mice. Addict Biol. 2011;16:386–392.
Panagopoulos VN, Ralevski E. The role of ghrelin in addiction: a review. Psychopharmacol (Berl). 2014;231:2725–2740.
Spencer SJ, Emmerzaal TL, Kozicz T, Andrews ZB. Ghrelin’s role in the hypothalamic-pituitary-adrenal axis stress response: implications for mood disorders. Biol Psychiatry. 2015;78:19–27.
Wittekind DA, Kluge M. Ghrelin in psychiatric disorders—a review. Psychoneuroendocrinology. 2015;52:176–194.
Leggio L. Role of the ghrelin system in alcoholism: acting on the growth hormone secretagogue receptor to treat alcohol-related diseases. Drug News Perspect. 2010;23:157–166.
Zallar LJ, Farokhnia, Tunstall BJ, Vendruscolo LF, Leggio L. The role of ghrelin in addictions. International review of neurobiology. 2017;136:89–119.
Parker CC, et al. Rats are the smart choice: rationale for a renewed focus on rats in behavioral genetics. Neuropharmacology. 2014;76:250–258.
Vengeliene V. The role of ghrelin in drug and natural reward. Addict Biol. 2013;18:897–900.
Ellenbroek B, Youn J. Rodent models in neuroscience research: is it a rat race? Dis Model Mech. 2016;9:1079–1087.
Clegg DJ, et al. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058.
Goldstein JL, et al. Surviving starvation: essential role of the ghrelin-growth hormone axis. Cold Spring Harb Symp Quant Biol. 2011;76:121–127.
Mann A, Thompson A, Robbins N, Blomkalns AL. Localization, identification, and excision of murine adipose depots. J Vis Exp. 2014;94:52174.
Azzout-Marniche D, et al. Obesity-prone high-fat-fed rats reduce caloric intake and adiposity and gain more fat-free mass when allowed to self-select protein from carbohydrate:fat intake. Am J Physiol Regul Integr Comp Physiol. 2016;310:R1169–R1176.
Lima ML, et al. A Novel Wistar Rat Model of Obesity-Related Nonalcoholic Fatty Liver Disease Induced by Sucrose-Rich Diet. J Diabetes Res. 2016;2016:9127076.
Aslani S, et al. The effect of high-fat diet on rat’s mood, feeding behavior and response to stress. Transl Psychiatry. 2015;5:e684.
Ikeda H, Shino A, Matsuo T, Iwatsuka H, Suzuoki Z. A new genetically obese-hyperglycemic rat (Wistar fatty). Diabetes. 1981;30:1045–1050.
Kovacs P, Voigt B, Berg S, Vogt L, Kloting I. WOK.1W rats. A potential animal model of the insulin resistance syndrome. Ann N Y Acad Sci. 1997;827:94–99.
Aleixandre de Artinano A, Miguel Castro M. Experimental rat models to study the metabolic syndrome. Br J Nutr. 2009;102:1246–1253.
Vendruscolo JC et al. Compulsive-like sufentanil vapor self-administration in the rat. Neuropsychopharmacology. 2017. Article in press. Doi# below.DOI: 10.1038/npp.2017.172
Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653.
Holtz NA, Carroll ME. Cocaine self-administration punished by intravenous histamine in adolescent and adult rats. Behav Pharmacol. 2015;26:393–397.
Cannon B., Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359.
Elattar S, Satyanarayana A. Can brown fat win the battle against white fat? J Cell Physiol. 2015;230:2311–2317.
Shuto Y, et al. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Invest. 2002;109:1429–1436.
Mano-Otagiri A, et al. Genetic suppression of ghrelin receptors activates brown adipocyte function and decreases fat storage in rats. Regul Pept. 2010;160:81–90.
Chondronikola M, Porter C, Malagaris I, Nella AA, Sidossis LS. Brown adipose tissue is associated with systemic concentrations of peptides secreted from the gastrointestinal system and involved in appetite regulation. Eur J Endocrinol. 2017;177:33–40.
Ma X, et al. Ablations of ghrelin and ghrelin receptor exhibit differential metabolic phenotypes and thermogenic capacity during aging. PloS ONE. 2011;6:e16391.
Lin L, Sun Y. Thermogenic characterization of ghrelin receptor null mice. Methods Enzymol. 2012;514:355–370.
Lin L, et al. The suppression of ghrelin signaling mitigates age-associated thermogenic impairment. Aging. 2014;6:1019–1032.
Acknowledgements
This work was supported by the National Institute on Drug Abuse Intramural Research Program (L.J.Z., B.J.T., C.T.R., Y.J.Z., Z.B.Y., E.L.G., G.F.K., L.F.V., B.K.H., L.L), the National Institute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research (L.J.Z., Y.J.Z., M.H., L.L.), and the National Institute of Mental Health Intramural Research Program (J.P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zallar, L.J., Tunstall, B.J., Richie, C.T. et al. Development and initial characterization of a novel ghrelin receptor CRISPR/Cas9 knockout wistar rat model. Int J Obes 43, 344–354 (2019). https://doi.org/10.1038/s41366-018-0013-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0013-5
This article is cited by
-
Impact of gene polymorphism of glutathione S-transferase and ghrelin as a risk factor in Egyptian women with gestational diabetes mellitus
Egyptian Journal of Medical Human Genetics (2022)
-
Involvement of the ghrelin system in the maintenance and reinstatement of cocaine-motivated behaviors: a role of adrenergic action at peripheral β1 receptors
Neuropsychopharmacology (2022)
-
Involvement of the ghrelin system in the maintenance of oxycodone self-administration: converging evidence from endocrine, pharmacologic and transgenic approaches
Molecular Psychiatry (2022)
-
Rat models of human diseases and related phenotypes: a systematic inventory of the causative genes
Journal of Biomedical Science (2020)
-
GPCR and Alcohol-Related Behaviors in Genetically Modified Mice
Neurotherapeutics (2020)