Abstract
The ferritin nanocage is an endogenous protein that exists in almost all mammals. Its hollow spherical structure that naturally stores iron ions has been diversely exploited by researchers in biotherapeutics. Ferritin has excellent biosafety profiles, and the nanosized particles exhibit rapid dispersion and controlled/sustained release pharmacokinetics. Moreover, the large surface-to-volume ratio and the disassembly/reassembly behavior of the 24 monomer subunits into a sphere allow diverse modifications by chemical and genetic methods on the surface and inner cage of ferritin. Here, we critically review ferritin and its applications. We (i) introduce the application of ferritin in drug delivery; (ii) present an overview of the use of ferritin in imaging and diagnosis for biomedical purposes; (iii) discuss ferritin-based vaccines; and (iv) review ferritin-based agents currently in clinical trials. Although there are no currently approved drugs based on ferritin, this multifunctional protein scaffold shows immense potential in drug development in diverse categories, and ferritin-based drugs have recently entered phase I clinical trials. This golden shortlist of recent developments will be of immediate benefit and interest to researchers studying ferritin and other protein-based biotherapeutics.
Similar content being viewed by others
Introduction
The exploitation of protein nanocarriers has played a vital role in advancing disease diagnosis and therapy1. Their nanometer size range allows advantageous properties that permit diverse modifications, such as surface functionalization, and enable the rapid dispersion of loaded drugs. Furthermore, protein nanocarriers have improved the targeted delivery and pharmacokinetic profiles of drug cargo. They are also cleared from circulation relatively slowly, which allows controlled/sustained drug release in targeted sites. Due to these advantages, diverse types of protein nanocarriers have been highlighted for their inherent biodegradability and ease of genetic modification compared to other types of nanocarriers2. In this review, we highlight ferritin nanocages.
Ferritin exists in almost all living organisms, participating in iron homeostasis by storing and releasing iron ions3. Ferritin has excellent biosafety profiles, and the nanosized particles exhibit rapid dispersion and controlled/sustained release pharmacokinetics. Moreover, the large surface-to-volume ratio and the disassembly/reassembly feature of the 24 monomer subunits into a sphere allow diverse modifications on the surface and inner cage of ferritin by chemical and genetic methods. Its hollow spherical structure that naturally stores iron ions has been diversely exploited by researchers in biotherapeutics4. Examples of drugs and agents loaded onto ferritin include chemotherapeutic agents, genes, fluorescent molecules, and various peptides that have been displayed on their surface. Optimizing drug loading efficiency is an ongoing task for all delivery vehicles; however, there have been diverse attempts to load various poorly soluble drugs into ferritin. Furthermore, the threefold symmetry axis of the ferritin structure has also allowed antigenic display in its correct conformation, a unique feature of ferritin compared to other particles5.
This review summarizes ferritin and its performance in various fields of medicine (Supplementary Fig. 1). The overall aim is to present a short list of the most interesting current and recent approaches to using ferritin in nanomedicine. Here, we critically review ferritin and its applications: (1) the application of ferritin in therapeutics; (2) the application of ferritin in imaging and diagnosis; (3) ferritin-based vaccines in immunotherapy; and (4) current and impending clinical trials of ferritin-based agents. This review of recent developments will be of immediate benefit and interest to researchers studying ferritin and other protein-based biotherapeutics.
Ferritins in biotherapeutics
The hollow spherical core of ferritin allows the loading of various cargo. This is generally achieved via the mineral pores on the surface or pH-mediated disassembly/reassembly of the nanocage. There have been many investigations, mainly in anticancer therapy that attempted the targeted delivery of chemotherapeutic agents to tumors using ferritin as a delivery vehicle. Most of these studies resulted in successful tumor growth inhibition, superior pharmacokinetic profiles and diminished adverse effects compared to the free drug in both in vitro and in vivo models. Substantial progress has been made in the development of ferritin nanocages for use in drug delivery for cancer therapy.
Doxorubicin-loaded ferritins have shown successful tumor growth inhibition in numerous mouse cancer models by many groups. In one example, Dox-loaded ferritin targeted and was internalized by transferrin receptor 1 (TfR1)-overexpressing tumor cells to mediate 10-fold higher intracellular levels compared to free doxorubicin6. Moreover, the encapsulation of paclitaxel in ferritin showed potent apoptosis of MDA-MB-231 breast cancer cells in in vivo models7. This is an example of insoluble drug delivery via ferritin, with specific targeting to tumor cells alleviating the adverse effects of this chemotherapeutic agent. Similarly, gemcitabine was loaded onto ferritin and coadministered for photothermal therapy, showing effective adjunctive therapy against breast cancer models7. Effective delivery of cisplatin was also achieved by ferritin, resulting in an improved therapeutic index of antiblastic therapy in an advanced, refractory melanoma model8. Specific delivery of chemotherapeutic agents to cancer cells by ferritin has resulted in enhanced therapeutic benefit to numerous types of cancer, with some successful in vivo results thus far (Table 1).
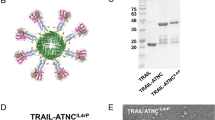
As mentioned above, the ferritin nanocage is composed of 24 monomer subunits, which allows the display of 24 separate peptides if arranged correctly by genetic modification. This has allowed diverse approaches in displaying various immune-stimulating peptides on the ferritin surface for the development of effective immunotherapeutic agents (Table 2). This is a unique advantage of ferritin nanocages and allows the delivery of various (and multiple) peptides via one-step modification9. For example, the trimeric peptide display arrangement of the surface of ferritin allows the optimal delivery of TRAIL peptides. More than one successful attempt has been made to deliver this apoptosis-inducing signal on the surface of ferritin nanocages10,11,12.
In another interesting work, the phagocytosis-inducing peptide SIRPα was displayed by doxorubicin-loaded ferritin13 to achieve an intrinsic vaccination effect. By the cross priming of effector CD8+ T cells, the simultaneous delivery of SIRPα and doxorubicin, an immunogenic cell death (ICD)-inducer, achieved potent tumor growth inhibition in a melanoma model and even against tumor rechallenge in a colon cancer model. This study attempted to trigger the presentation of cancer cell neoantigens to the host immune system, facilitating the persistent amplification of antitumour T cells, which resulted in an especially interesting and effective therapeutic approach. There are also other interesting approaches using ferritin in addition to the abovementioned studies, such as a study by Seo et al. that proposed a thrombolytic ferritin expressing multivalent clot-targeting peptides and fibrin degradation enzymes for coadministration with chemotherapy14,15. Similarly, a study by Lee et al. used γ-carboxyglutamic acid of protein C (PC-Gla) and thrombin receptor agonist peptide (TRAP) to treat acute inflammatory sepsis in in vivo mouse models16.
Ferritins in imaging and diagnosis
Ferritin nanocages have been readily modified to develop diagnostic agents for various imaging methods (computer tomography, CT/magnetic resonance imaging MRI). Fluorescent molecules can be incorporated or loaded as cargo at the same time as targeting peptides on the ferritin surface for targeting disease biomarkers17. This would allow multimodal imaging techniques for ferritin-based agents with enhanced diagnostic accuracy, and the ferritin-based agents developed are listed below (Table 3).
For example, magnetoferritin probes consisting of iron oxide and 125I radionuclides on the ferritin surface were developed for the multimodal imaging of tumors by Zhao et al.18. Moreover, a study by Huang et al. developed near-infrared dye IR820-loaded ferritin for fluorescence and photoacoustic photothermal therapy with high imaging contrast19. Another study by Crich et al. developed gadolinium-loaded ferritin for contrast MRI, which allowed the imaging of tumoral endothelial cells20.
More common fluorescent molecules, such as red fluorescent protein (RFP), have been loaded in a study by Lee et al., where tumor-specific antigens were simultaneously delivered to the lymph nodes in mouse models21. Another example is a study by Lin et al. that developed hybrid ferritin probes for tumor cell-targeted near-infrared fluorescence (NIRF) imaging22. Similarly, indocyanine green (ICG)-loaded ferritin was developed by Sitia et al. for tumor-specific imaging that showed clinical relevance for fluorescence imaging-guided surgery of cancer23. In addition, fluorescent Cy5.5 was loaded onto magnetic ferritin, which targeted vascular macrophages in imaging in vivo inflammation24. As shown by these examples, ferritin is a multifunctional platform that can also target specific cells/biomarkers.
Ferritin-based vaccines in immunotherapy
Ferritin-based vaccines have attracted considerable interest due to their potency and safety25. Conventional vaccines composed of inactivated viruses or organisms carry the potential risk of triggering reversion, and thus, attempts to develop more immunogenic yet safe vaccines continue26. Antigen display on the ferritin surface has many desirable features, such as the uniform presentation of 24 epitopes, as well as monodispersity and thermal and pH stability of the ferritin nanocage. Furthermore, the particle-mediated delivery of peptides has been shown to trigger more potent stimulation than soluble peptides27. Ferritin nanocages, due to their size (10~12 nm), can be readily taken up by dendritic cells (DCs) for migration to the lymph node to augment cellular and humoral immune responses. Due to these numerous advantages, ferritin-based vaccines have proven especially potent and can be applied not only to infectious diseases but also to cancer vaccines and vaccines for autoimmune diseases.
Representative ferritin-based vaccines target influenza, SARS-CoV-2 and Epstein‒Barr viruses, and some have entered phase I clinical trials26,28. Ferritin-based vaccines have proven biocompatible yet immunogenic with no significant adverse effects. However, the challenging features of ferritin-based vaccine development are nanoparticle heterogeneity, inadequate folding of antigens and intersubunit interactions resulting in antigen interference. Since antigens are encoded onto the ferritin protein scaffold, the self-assembled expression and purification of ferritin-based vaccines still require careful optimization. The selected clinical trials of ferritin nanocages currently in vaccine development are listed below (Table 4).
Ferritins in clinical trials
Clinical trials with ferritin-based vaccines are in the early stages (Table 5). One example is the work of Kanekiyo et al.29, in which trimeric hemagglutinin (HA) was fused to the 3-fold axis of ferritin, giving the display of eight trimeric viral spikes. The ferritin-HA showed a potent immunological effect (10-fold higher antibody titers) compared to an inactivated vaccine. The promising results obtained with this design have motivated the development of three vaccines against influenza to be tested in Phase 1 clinical trials (ClinicalTrials.gov ID NCT03186781, NCT03814720, and NCT04579250)29,30.
Positive results have been obtained from the first trial for influenza, NCT03186781, where the seroconversion rates were 40% or 90%, and the 50% inhibitory concentration (IC50) titers were 1 × 103 and 3 × 103 for monotherapy and coadministration with an influenza DNA vaccine, respectively31. No significant side effects were reported. The ferritin-HA vaccine was followed by the development of other ferritin-based vaccines targeting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although no results have been posted yet, a single administration of this vaccine was effective in mouse models, and this study has also entered phase I clinical trials (ClinicalTrials.gov ID NCT04784767)32.
The third ferritin-based vaccine targets Epstein‒Barr virus (EBV) and has also entered a phase 1 clinical trial (ClinicalTrials.gov ID NCT04645147). In this vaccine, the surface of ferritin is decorated with gp350 (glycoprotein 350/220 of EBV), and the neutralization capacity was reported to be significantly higher than that of soluble gp350 in mouse models33.
Conclusion
Protein nanocarriers contribute numerous advantages to the field of disease diagnosis and drug development, and current approaches in diverse categories of biotherapeutics, immunotherapy, and vaccines will in no doubt provide therapeutic benefit. Ferritin has excellent biocompatibility and biodegradability and provides many possibilities for modification. The subunit structure of ferritin allows the uniform display of 24 peptides on its surface, which can be achieved by one-step genetic modification or direct conjugation via chemical methods. Furthermore, the triggering of a more potent response by the particle-mediated delivery of peptides is a well-known phenomenon in peptide delivery, as is the triggering of immunomodulatory responses. Additionally, the hollow cage structure allows the delivery of various poorly soluble or cytotoxic drugs by disassembly/reassembly or mineralization through surface pores. Ferritin is an all-in-one multifunctional protein scaffold.
The major strength of ferritin nanocages in nanomedicine is in vaccine development. Three vaccines against influenza have entered phase I clinical trials, and one has provided positive results. The efficacy of ferritin-based vaccines has triggered investigations into optimizations for developing other ferritin-based vaccines in clinical trials. Ferritin holds great potential in disease diagnosis, prevention and therapy.
Data availability
All data are included in this published article.
References
Lee, E. J., Lee, N. K. & Kim, I. S. Bioengineered protein-based nanocage for drug delivery. Adv. Drug Deliv. Rev. 106, 157–171 (2016).
Wang, Z. et al. Functional ferritin nanoparticles for biomedical applications. Front. Chem. Sci. Eng. 11, 633–646 (2017).
Jutz, G., van Rijn, P., Santos Miranda, B. & Boker, A. Ferritin: a versatile building block for bionanotechnology. Chem. Rev. 115, 1653–1701 (2015).
Truffi, M. et al. Ferritin nanocages: a biological platform for drug delivery, imaging and theranostics in cancer. Pharmacol. Res. 107, 57–65 (2016).
Song, N. et al. Ferritin: a multifunctional nanoplatform for biological detection, imaging diagnosis, and drug delivery. Acc. Chem. Res. 54, 3313–3325 (2021).
Zhen, Z. et al. Ferritin nanocages to encapsulate and deliver photosensitizers for efficient photodynamic therapy against cancer. ACS Nano 7, 6988–6996 (2013).
Zhang, Q. et al. Inlaying radiosensitizer onto the polypeptide shell of drug-loaded ferritin for imaging and combinational chemo-radiotherapy. Theranostics 9, 2779–2790 (2019).
Falvo, E. et al. Antibody-drug conjugates: targeting melanoma with cisplatin encapsulated in protein-cage nanoparticles based on human ferritin. Nanoscale 5, 12278–12285 (2013).
Palombarini, F., Di Fabio, E., Boffi, A., Macone, A. & Bonamore, A. Ferritin nanocages for protein delivery to tumor cells. Molecules 25, 825 (2020).
Kih, M. et al. Designed trimer-mimetic TNF superfamily ligands on self-assembling nanocages. Biomaterials 180, 67–77 (2018).
Yoo, J. D. et al. Designed ferritin nanocages displaying trimeric TRAIL and tumor-targeting peptides confer superior anti-tumor efficacy. Sci. Rep. 10, 19997 (2020).
Je, H. et al. Overcoming therapeutic efficiency limitations against TRAIL-resistant tumors using re-sensitizing agent-loaded trimeric TRAIL-presenting nanocages. J. Control. Rel. 331, 7–18 (2021).
Lee, E. J. et al. Nanocage-therapeutics prevailing phagocytosis and immunogenic cell death awakens immunity against cancer. Adv. Mater. https://doi.org/10.1002/adma.201705581 (2018).
Seo, J. et al. A targeted ferritin-microplasmin based thrombolytic nanocage selectively dissolves blood clots. Nanomedicine 14, 633–642 (2018).
Seo, J. et al. Fibrinolytic nanocages dissolve clots in the tumor microenvironment, improving the distribution and therapeutic efficacy of anticancer drugs. Exp. Mol. Med. 53, 1592–1601 (2021).
Lee, W. et al. A double-chambered protein nanocage loaded with thrombin receptor agonist peptide (TRAP) and gamma-carboxyglutamic acid of protein C (PC-Gla) for sepsis treatment. Adv. Mater. 27, 6637–6643 (2015).
Zhen, Z., Tang, W., Todd, T. & Xie, J. Ferritins as nanoplatforms for imaging and drug delivery. Expert Opin. Drug Deliv. 11, 1913–1922 (2014).
Zhao, Y. et al. Bioengineered magnetoferritin nanoprobes for single-dose nuclear-magnetic resonance tumor imaging. ACS Nano 10, 4184–4191 (2016).
Huang, P. et al. Dye-loaded ferritin nanocages for multimodal imaging and photothermal therapy. Adv. Mater. 26, 6401–6408 (2014).
Geninatti Crich, S. et al. Magnetic resonance visualization of tumor angiogenesis by targeting neural cell adhesion molecules with the highly sensitive gadolinium-loaded apoferritin probe. Cancer Res. 66, 9196–9201 (2006).
Lee, B. R. et al. Engineered human ferritin nanoparticles for direct delivery of tumor antigens to lymph node and cancer immunotherapy. Sci. Rep. 6, 35182 (2016).
Lin, X. et al. Hybrid ferritin nanoparticles as activatable probes for tumor imaging. Angew. Chem. Int. Ed. Engl. 50, 1569–1572 (2011).
Sitia, L. et al. Development of tumor-targeted indocyanine green-loaded ferritin nanoparticles for intraoperative detection of cancers. ACS Omega 5, 12035–12045 (2020).
Terashima, M. et al. Human ferritin cages for imaging vascular macrophages. Biomaterials 32, 1430–1437 (2011).
Sung, H. D., Kim, N., Lee, Y. & Lee, E. J. Protein-based nanoparticle vaccines for SARS-CoV-2. Int. J. Mol. Sci. 22, 13445 (2021).
Rodrigues, M. Q., Alves, P. M. & Roldao, A. Functionalizing ferritin nanoparticles for vaccine development. Pharmaceutics 13, 1621 (2021).
Smith, D. M., Simon, J. K. & Baker, J. R. Jr. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 13, 592–605 (2013).
Kim, S. A. et al. A multivalent vaccine based on ferritin nanocage elicits potent protective immune responses against SARS-CoV-2 mutations. Int. J. Mol. Sci. 23, 6123 (2022).
Kanekiyo, M. et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 499, 102–106 (2013).
Yassine, H. M. et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 21, 1065–1070 (2015).
Houser, K. V. et al. Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: a phase 1 trial. Nat. Med. 28, 383–391 (2022).
Joyce, M. G. et al. SARS-CoV-2 ferritin nanoparticle vaccines elicit broad SARS coronavirus immunogenicity. Cell Rep. 37, 110143 (2021).
Kanekiyo, M. et al. Rational design of an Epstein-Barr virus vaccine targeting the receptor-binding site. Cell 162, 1090–1100 (2015).
Fantechi, E. et al. A smart platform for hyperthermia application in cancer treatment: cobalt-doped ferrite nanoparticles mineralized in human ferritin cages. ACS Nano 8, 4705–4719 (2014).
Cheng, X. et al. TfR1 binding with H-ferritin nanocarrier achieves prognostic diagnosis and enhances the therapeutic efficacy in clinical gastric cancer. Cell Death Dis. 11, 92 (2020).
Li, M., Wu, D., Chen, Y., Shan, G. & Liu, Y. Apoferritin nanocages with Au nanoshell coating as drug carrier for multistimuli-responsive drug release. Mater. Sci. Eng. C. Mater. Biol. Appl. 95, 11–18 (2019).
Yao, H. et al. Dual-functional carbon dot-labeled heavy-chain ferritin for self-targeting bio-imaging and chemo-photodynamic therapy. J. Mater. Chem. B 6, 3107–3115 (2018).
Lin, C. Y. & Shieh, M. J. Near-infrared fluorescent dye-decorated nanocages to form grenade-like nanoparticles with dual control release for photothermal theranostics and chemotherapy. Bioconjug. Chem. 29, 1384–1398 (2018).
Du, B. et al. A self-targeting, dual ROS/pH-responsive apoferritin nanocage for spatiotemporally controlled drug delivery to breast cancer. Biomacromolecules 19, 1026–1036 (2018).
Geninatti Crich, S. et al. Targeting ferritin receptors for the selective delivery of imaging and therapeutic agents to breast cancer cells. Nanoscale 7, 6527–6533 (2015).
Kuruppu, A. I. et al. An apoferritin-based drug delivery system for the tyrosine kinase inhibitor gefitinib. Adv. Healthc. Mater. 4, 2816–2821 (2015).
Jeon, I. S. et al. Anticancer nanocage platforms for combined immunotherapy designed to harness immune checkpoints and deliver anticancer drugs. Biomaterials 270, 120685 (2021).
Kim, G. B. et al. Design of PD-1-decorated nanocages targeting tumor-draining lymph node for promoting T cell activation. J. Control. Rel. 333, 328–338 (2021).
Kim, S. et al. Double-chambered ferritin platform: dual-function payloads of cytotoxic peptides and fluorescent protein. Biomacromolecules 17, 12–19 (2016).
Jiang, B. et al. Biomineralization synthesis of the cobalt nanozyme in SP94-ferritin nanocages for prognostic diagnosis of hepatocellular carcinoma. ACS Appl. Mater. Interfaces 11, 9747–9755 (2019).
Zhang, W. et al. Prussian blue modified ferritin as peroxidase mimetics and its applications in biological detection. J. Nanosci. Nanotechnol. 13, 60–67 (2013).
Szabo, I., Crich, S. G., Alberti, D., Kalman, F. K. & Aime, S. Mn loaded apoferritin as an MRI sensor of melanin formation in melanoma cells. Chem. Commun. 48, 2436–2438 (2012).
Nasrollahi, F. et al. Incorporation of graphene quantum dots, iron, and doxorubicin in/on ferritin nanocages for bimodal imaging and drug delivery. Adv. Therapeutics https://doi.org/10.1002/adtp.201900183 (2020).
Li, K. et al. Multifunctional ferritin cage nanostructures for fluorescence and MR imaging of tumor cells. Nanoscale 4, 188–193 (2012).
Lin, X. et al. Chimeric ferritin nanocages for multiple function loading and multimodal imaging. Nano Lett. 11, 814–819 (2011).
Yang, M. et al. Dragon fruit-like biocage as an iron trapping nanoplatform for high efficiency targeted cancer multimodality imaging. Biomaterials 69, 30–37 (2015).
Uchida, M. et al. Targeting of cancer cells with ferrimagnetic ferritin cage nanoparticles. J. Am. Chem. Soc. 128, 16626–16633 (2006).
Powell, A. E. et al. A single immunization with spike-functionalized ferritin vaccines elicits neutralizing antibody responses against SARS-CoV-2 in mice. ACS Cent. Sci. 7, 183–199 (2021).
Reinshagen, C. et al. CRISPR-enhanced engineering of therapy-sensitive cancer cells for self-targeting of primary and metastatic tumors. Sci. Transl. Med. 10, eaao3240 (2018).
Kanekiyo, M. et al. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat. Immunol. 20, 362–372 (2019).
Kamp, H. D. et al. Design of a broadly reactive Lyme disease vaccine. NPJ Vaccines 5, 33 (2020).
Acknowledgements
This work was supported by the National Research Foundation (NRF) of Korea, funded by the Ministry of Science (No. 2017R1A3B1023418, 2021R1A6A3A01088199) and the National Cancer Center.
Author information
Authors and Affiliations
Contributions
N.K.L., conceptualization and writing; S.C., conceptualization and writing; and I.-S.K., writing – reviewing and editing and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, N.K., Cho, S. & Kim, IS. Ferritin – a multifaceted protein scaffold for biotherapeutics. Exp Mol Med 54, 1652–1657 (2022). https://doi.org/10.1038/s12276-022-00859-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-022-00859-0