Abstract
Cancer cachexia syndrome is a major cause of morbidity and mortality in cancer patients in the advanced stage. It is a devastating disorder characterized by nutritional impairment, weakness, and wasting, and it affects treatment success and quality of life. Two major symptoms of cancer cachexia are anorexia and weight loss. Weight loss in cachexia is not reversed through increased food intake, suggesting that anorexia and weight loss in cancer patients are regulated by independent molecular mechanisms. Although the wasting phenotype mostly occurs in skeletal muscle and adipose tissue, other organs, such as the brain, liver, pancreas, heart, and gut, are also involved in cachexia. Thus, cachexia is a multiorgan syndrome. Although the molecular basis of cancer cachexia-induced weight loss is known, the mechanism underlying anorexia is poorly understood. Here, we highlight our recent discovery of a new anorexia mechanism by which a tumor-derived humoral factor induces cancer anorexia by regulating feeding-related neuropeptide hormones in the brain. Furthermore, we elucidated the process through which anorexia precedes tissue wasting in cachexia. This review article aims to provide an overview of the key molecular mechanisms of anorexia and tissue wasting caused by cancer cachexia.
Similar content being viewed by others
Introduction
Cancer cachexia is defined as a multifactorial metabolic syndrome occurring in advanced cancer patients that negatively affects quality of life and leads to early death1,2,3,4,5. Anorexia and weight loss are two major symptoms of cancer cachexia6. Weight loss occurs because of the loss of skeletal muscle and fat tissue, and cancer anorexia is caused by a failure of appetite signals2,7. Cancer cachexia not only decreases the quality of life of cancer patients but also has negative effects during the chemotherapy process8. Therefore, understanding the underlying molecular mechanisms is critical to ensure an accurate diagnosis of cancer cachexia and to design treatments for cancer patients. Cancer cachexia is detected in more than 80% of gastrointestinal or pancreatic cancer patients and is less common in lymphoma or breast cancer patients9,10. Anorexia and weight loss induced by cancer cachexia are the direct causes of death in up to 20% of all cancer patients11,12. The failure of appetite signaling caused by cancer anorexia differs from malnutrition or hunger; it is caused by metabolic abnormalities such as increased basal metabolism, decreased fat production, increased fat breakdown, and increased muscle protein breakdown13. Anorexia can negatively impact cancer patients through weight loss and adipose tissue wasting, but weight loss does not always occur with cancer anorexia14. In addition, the weight loss is not recovered through increased food intake, suggesting that anorexia and weight loss in cancer cachexia patients are controlled by independent molecular mechanisms1,2,15.
The major cause of cancer cachexia is the deterioration of normal tissue function caused by inflammatory cytokines, which are released in excessive amounts by tumors16. Tumor growth triggers an inflammatory response around the tumor, which induces the secretion of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1β17,18. These secreted tumor-derived humoral cytokines play an essential role in interorgan/tissue communication. The interorgan communication that underlies the systemic coordination and integration between organs and tissues is important under normal and pathological conditions, such as cancer and metabolic syndrome19. Tumors elicit an inflammatory response that leads to metabolic dysfunction in muscle and fat, as well as in the brain, by causing an inflammatory cytokine burst20,21. Recent data indicate that various factors other than inflammatory cytokines are produced and secreted in a cancer cachexia model. In a cancer model, the secretion of transforming growth factor-beta (TGF-β) and parathyroid-hormone related protein (PTHrP) causes the cachexia phenotype by inducing an imbalance of muscle tissue and fat metabolism22,23,24. Exposure to a neutralizing antibody against PTHrP alleviates cancer cachexia25.
In a statistical analysis of 1307 cancer patients, cachexia was divided according to severity into the pre-cachexia stage, with a weight loss of <10%, and the cachexia stage, with a weight loss of >10%. In addition to weight loss, loss of appetite, fatigue, and early satiety are important factors involved in the pre-cachexia stage and the cachexia stage26. However, the relationship between cancer anorexia and weight loss during cancer cachexia remains unclear. Furthermore, the pathological mechanisms, criteria for diagnosis, and applicable blood biomarkers for cancer cachexic patients are not yet available. This review examines the molecular mechanisms underlying cancer cachexia by analyzing the two major processes, namely, cancer anorexia and tissue wasting. In addition, we examined the relationship between anorexia and wasting in cancer cachexia.
Cancer anorexia
Anorexia (loss of appetite) is a major symptom of cachexia that develops in most cancer patients27. Among the multiple causes of cancer anorexia28, the primary cause is the increase in substances released by the tumor, such as pro-inflammatory cytokines or lactate21. Alternatively, there are many peripheral causes of anorexia, including dysphagia, alterations in gastrointestinal functions, hypoxia, alterations in nutrients by the tumor, and alterations in food intake due to the release of peripheral hormones21. Chemotherapy also causes anorexia. Chemotherapy peripherally affects taste perception and may cause nausea, vomiting, and dysgeusia. However, the underlying mechanism remains unclear29. In addition to these peripheral causes, the central cause of anorexia may be related to multiple alterations in various neurotransmitters or neuropeptides, which can lead to depression, pain, and decreased appetite21.
Cytokines are peptide hormones released from tumor tissues or the immune system. Pro-inflammatory cytokines, such as TNFα, IL-1, and IL-6, are increased in tumors30,31. Studies show that these cytokines are the direct cause of reduced food intake in cancer patients32,33,34. IL-1 is secreted by T cells and macrophages, and it is the most potent anorectic factor. IL-1 can decrease the size, duration, and frequency of meal intake35, and antibodies against IL-1 restore food intake in tumor-bearing mice36. TNFα produced by macrophages and monocytes is increased in tumors in mice and induces anorexia via central and peripheral effects37,38,39.
We recently reported that a new type of cytokine, Dilp8/INSL3, induces anorexia in cancer patients40. Drosophila Dilp8, the mammalian homolog of INSL3, is released from tumor tissues and systemically controls the expression of feeding-associated neuropeptides through the Lgr3/Lgr8 receptor in the brain. We demonstrated that the regulation of INSL3 in cancer anorexia is conserved in mice. The study showed that the secretion of INSL3 into cell culture medium and serum INSL3 levels are increased in a mouse cancer model and human cancer patients, respectively. Direct injection of INSL3 into the brain reduces food intake in wild-type mice, whereas no changes are observed in response to a peripheral injection of INSL340. The peripheral injection of INSL3 in LLC tumor-bearing mice initially showed no changes in food intake, but these mice ultimately developed anorexia. These findings suggest that various systemic changes are necessary for INSL3 delivery to the hypothalamus. Recent studies of cancer cachexia using the fly cancer model reported a dramatic increase in the expression of three tumor-derived cytokines, Dilp8, ImpL2, and Upd240,41,42. ImpL2 is a Drosophila homolog of mammalian insulin-growth factor binding protein (IGFBP), and it causes organ wasting independent of food consumption. Another factor, Upd2, does not cause organ wasting or anorexia in flies, whereas mouse IL-6 (Upd2 ortholog) induces white adipose tissue (WAT) browning under cachexia conditions43. Tumor-derived IL-6/Upd2 induces noncell-autonomous autophagy around tumor tissues44.
In the central nervous system, tumors cause changes in neurotransmitters and neuropeptides that alter feeding. The hypothalamus, which is a critical region for cachexia/anorexia development, regulates both food intake and body energy expenditure45. At the central level of the hypothalamus, peripheral signals regulate orexigenic factors, such as neuropeptide Y (NPY) and agouti-related protein (AgRP), through a food intake-promoting axis or anorexigenic factors, such as pro-opiomelanocortin (POMC) precursor. POMC is involved in the production of the melanocyte-stimulating hormone α-MSH46. The main appetite-stimulating neuropeptides NPY and AgRP originate from the same arcuate nucleus neurons near the hypothalamic median eminence46,47. NPY binds to postsynaptic Y1 and Y5 receptors, which release AgRP46. In a parallel system, POMC neurons from the arcuate nucleus innervate both the paraventricular and lateral hypothalamus. POMC reduces food intake by binding to postsynaptic melanocortin receptor 4, which releases secondary effectors such as thyrotropin-releasing hormone, corticotropin-releasing hormone (CRH), and oxytocin48,49. In addition, leptin is the major appetite-suppressing hormone that activates POMC gene expression and inhibits NPY expression. Leptin is produced by WAT and exerts anorexigenic effects through CRH50,51. However, there is currently no evidence supporting a role for leptin in cancer anorexia. Although the circulating leptin level in cancer cachexia patients is decreased, it is not associated with a compensatory increase in food intake52.
In recent work from our group, we showed that the anorexigenic factor nesfatin-1 is involved in inducing cancer anorexia40. The Nesfatin-1 peptide consists of 82 amino acids produced from the nucleobindin 2 (NUCB2) precursor. It is expressed in the hypothalamic region and reduces food intake in mammals53. The NUCB2 precursor protein is expressed in the hypothalamic region, including the supraoptic nucleus, lateral hypothalamic area, arcuate nucleus, paraventricular nucleus53, and parabranchial nucleus (PBN)54. Recent findings indicate that calcitonin gene-related peptide-positive neurons in the PBN region contribute to cancer-induced appetite suppression in LLC tumor-implanted mice55. We validated the anorectic effect of NUCB1 (NUCB2 homolog in Drosophila) in the Drosophila system and proposed that NUCB1-expressing neurons in the brain are important for anorexigenic regulation under normal and malignant conditions. However, the detailed function of the Nesfatin-1 receptor remains to be discovered. Thus, further studies are needed to examine the receptor for Nesfatin-1 and to elucidate the precise mechanism by which Nesfatin-1 induces anorexia.
Tissue wasting in skeletal muscle and adipose tissue
Cancer cachexia is considered a disorder of energy balance that occurs when energy intake decreases and/or when energy expenditure increases56,57,58,59. The balance of energy expenditure can depend on the type and stage of tumors. Increased energy expenditure is a possible cause of wasting syndrome that leads to involuntary weight loss. One example of a major molecular mechanism that contributes to energy expenditure is the “Cori cycle”. The Warburg effect results in the production of an excess amount of lactate by tumors due to anaerobic glycolysis. The Cori cycle mediates the hepatic uptake of excess lactate produced by tumors and its conversion to glucose. It then returns to the circulation and is reused for glycolysis. This inefficient recycling of lactate requires high energy because the conversion of glucose into lactate generates less ATP than the amount needed to produce glucose from lactate60. Another wasting mechanism identified in a mouse cancer model is decreased mitochondrial ATP synthesis in skeletal muscles61. In the skeletal muscle mitochondria of cancer patients, decreased oxidative capacity and membrane fluidity impair mitochondrial function62. However, the biochemical mechanisms of wasting remain elusive.
Wasting during cancer cachexia occurs mostly in skeletal muscle and adipose tissue. Skeletal muscle is an essential organ for various biological processes, including body movement and respiration. The maintenance of muscle homeostasis requires a balance between protein synthesis and degradation, and a decrease in protein synthesis or excessive degradation results in muscle wasting63. During tumor progression, cytokines or other factors that regulate the anabolic and catabolic system network are disrupted64. Studies suggest that the circulating level of the anabolic factor insulin-like growth factor-1 (IGF-1) is decreased, and insulin resistance occurs in the cancer cachexia model65,66,67,68,69,70. In contrast, the production of pro-cachexic or pro-inflammatory factors, such as TNF-α71,72 and IL-173,74,75,76, is increased, thereby promoting catabolism. These cytokines are involved in two well-known signaling pathways: the nuclear factor-κB (NF-κB) and p38 MAPK pathways77. In the NF-κB pathway, inflammatory mediators, such as myostatin or proteolysis-inducing factors, cause the transcriptional upregulation of genes encoding ubiquitin ligases (muscle RING finger-containing protein 1 (MURF-1) and muscle atrophy F-box protein). These ligases mediate proteolysis of myofibrillar proteins and inhibit protein synthesis77. Accordingly, inhibition of NF-κB by blocking the upregulation of MURF-1 significantly decreases muscle weight loss in a cancer cachexia model78,79. In the p38 MAPK pathway, mediators activate the p38 and MAPK cascades, which increase the activity of caspases, leading to apoptosis77.
Recent studies have examined the mechanism of insulin resistance using Drosophila cancer models41,42. In these cachexia models, the expression of ImpL2, the Drosophila homolog of mammalian IGFBP, is increased in tumor tissues, which disrupts both insulin and IGF-1 signaling. Secretion of ImpL2 by tumor tissues directly promotes insulin resistance in peripheral organs, such as muscle, fat, and ovaries, and ultimately causes systemic wasting41,42. In addition, direct exposure of C2C12 muscle cells to IGFBP3 induces muscle cell wasting, and the wasting effect is improved by IGFBP3 knockdown or IGFBP3 antibody neutralization80.
Growth differentiation factor-15 (GDF15), a TGF-β superfamily member, was recently highlighted as a biomarker for the early diagnosis of cachexia81,82,83,84. Antibody-mediated inhibition of the GDF15 receptor reverses cancer cachexia in mice, suggesting a novel function of the inflammatory growth factor GDF1585. This study identified a correlation between the circulating level of GDF15 and cachexia and showed that GDF15-induced body weight loss is mediated by the GDNF family receptor-A-like (GFRAL)-Ret proto-oncogene (RET) signaling complex in brainstem neurons85. In addition, a study showed that activation of the GFRAL-RET pathway induces the expression of genes involved in lipid metabolism and adipose tissues, and GDF15-mediated weight loss is prevented in adipose triglyceride lipase knockout mice85. This breakthrough study showed that inhibition of the GDF15-GFRAL pathway is a novel strategy for the treatment of cachexia in a mouse model, and this approach is currently being evaluated in phase I clinical trials.
In parallel with skeletal muscle loss, adipose tissue wasting commonly accompanies cancer cachexia. WAT wasting occurs through three different processes86. First, lipolytic activity is increased, leading to the release of high levels of free fatty acids and glycerol86. This event is involved in the activation of hormone-sensitive lipase. This lipase stimulates lipolysis and is related to the increased circulation of the lipid mobilization-promoting adipokine ZAG in adipose tissue71,87. Second, decreased activity of lipoprotein lipase, which cleaves triacylglycerol into glycerol and fatty acids, disrupts lipid uptake86. Last, de novo lipogenesis in WAT is reduced in cancer cachectic patients or mice; thus, triacylglycerol synthesis and lipid deposition are decreased86. WAT changes to brown adipose tissue (also called “browning”) during cancer cachexia24,43. The browning process is caused by high expression of uncoupling protein 1 (UCP1) in mitochondria, which switches the electrochemical gradient from ATP synthesis to thermogenesis88. This results in increased lipid mobilization and energy expenditure, which are common in cancer cachectic patients24. Several factors, such as IL-6 and PTHrP, which are derived from tumor or immune cells, are involved in the regulation of UCP124,25.
The wasting of skeletal muscle and adipose tissue is a critical factor that contributes to cancer cachexia by stimulating lipolysis and increasing energy expenditure. Additional features that are characteristic of cancer cachexia and occur in other organ systems will be discussed below.
Cancer anorexia precedes wasting
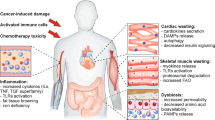
Cancer cachexia can be divided into three clinical stages according to weight loss: pre-cachexia (<10% weight loss), cachexia (≥10% weight loss), and refractory cachexia2 (Fig. 1). The frequency, penetrance, and severity of cachexia are highly variable and depend on the type and stage of tumors. Specific biomarkers for the diagnosis or treatment of early-stage cachexia have not been identified to date. Anorexia and weight loss are regulated by independent mechanisms, and cancer induces cachexia with body weight loss or the wasting phenotype independent of food intake. Despite several attempts to classify and categorize the stages of cachexia in cancer patients, there is limited information on the relationship between weight loss and anorexia, including the preference of the occurrence of the two symptoms.
In a recent study, in addition to elucidating the mechanism of cancer anorexia in both Drosophila and mammalian systems, we discovered that the process of anorexia precedes the onset of wasting40 (Fig. 1). Overexpression of the oncogene yki in the Drosophila cancer model demonstrated that the anorexia phenotype appeared early, as it started at 5 days. This finding is consistent with the fact that most cancer patients show a loss of appetite at the pre-cachexia stage, whereas the organ-wasting phenotype appears at 15–20 days. This finding was demonstrated in the Drosophila cancer model as well as in the mouse cancer model. In C26 tumor-bearing mice, anorexia induced by INSL3 secretion started on Day 11, although no body weight changes were observed in this time period. Alteration of body weight and muscle/fat atrophy markers occurs at 2–3 weeks after tumor cell implantation, suggesting that anorexia develops prior to the process of wasting in a mouse cancer model. Although the inhibition of Dilp8 does not restore the cancer-induced organ-wasting phenotype41, Dilp8 inhibition rescues the cancer anorexia phenotype40. These findings suggest that Dilp8 and ImpL2 play critical roles in regulating cancer anorexia and wasting, respectively.
The significance of identifying INSL3/Dilp8 as a new tumor-derived anorexia factor was described in a recent commentary by Wang et al.89. These findings highlight our discovery of a new tumor-derived factor in the brain that induces anorexia. Previous studies have focused on cancer-induced cachexia in advanced or metastatic stages. As noted by Wang et al.89 the finding that INSL3 functions at early stages indicates that cancer patients exhibit anorexia ahead of involuntary weight loss. In addition, recent evidence suggests that other systemic effects induced by cancer, such as premetastatic niche formation, occur in the early stages of cancer before progression to metastatic disease90. Further studies elucidating the relationship between anorexia and other systemic effects will improve our understanding of the early stages of cancer progression89. Therefore, targeting tumor-derived factors related to anorexia or other systemic effects in the early stages could be beneficial for preventing or treating cancer cachexia.
Cancer cachexia as a multiorgan syndrome
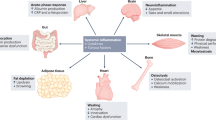
Weight loss that occurs in skeletal muscles accounts for >40% of the cachexia phenotype. However, recent studies suggest that other tissues, such as adipose tissue, brain, liver, pancreas, heart, and gut, are also involved in cachexia development and are related to muscle wasting (Fig. 2). In addition to the involvement of adipose tissue and the brain in the cachectic process, recent evidence suggests that the gut microbiota is involved in cancer cachexia91,92. In the proposed gut microbiota and skeletal muscle axis, the gut microbiota generates metabolites that are delivered to skeletal muscle, leading to increased energy expenditure in muscle cells93,94.
Another link between the gastrointestinal tract and skeletal muscle wasting is ghrelin. Ghrelin is a 28-amino acid peptide that is mainly secreted by the stomach and increases food intake through a nitric oxide-dependent mechanism95. Although circulating ghrelin levels are elevated in cancer patients, “ghrelin resistance” is also observed in cancer patients69,96. However, the administration of a long-acting ghrelin analog increases food intake and weight gain in tumor-implanted rats97. Ghrelin also directly influences muscle cells by blocking the increased protein degradation that is promoted by catabolic cytokines98. Based on its orexigenic properties, ghrelin has recently been investigated in clinical studies as a treatment for patients with anorexia or those undergoing chemotherapy. Studies show that ghrelin increases food intake and meal appreciation in cancer anorexia patients99. In patients undergoing chemotherapy, ghrelin improves food intake and appetite, thereby alleviating the effects of anorexia and nausea100. Glucagon-like peptide 1 (GLP-1) is another gut hormone that promotes insulin secretion and has been extensively explored for the treatment of metabolic diseases, such as obesity and type 2 diabetes. Despite its well-known role in metabolism, the link between GLP-1 and cachexia has not been studied in detail. Cachexia patients often show changes in glucose homeostasis and insulin sensitivity. The GLP-1 agonist and insulin sensitizer exendin-4 partially rescued cachexia in tumor-bearing rats101. Additional recent evidence suggests that brainstem GLP-1 signaling contributes to body weight loss and lean/fat mass in a cachexia rat model102. These findings suggest that gut hormones are good candidates for the treatment of cancer cachexia.
Glucose intolerance was first identified in cancer patients in 1919. Higher endogenous glucose production with increased gluconeogenesis is associated with cancer cachexia103. Insulin resistance is another common feature of cancer cachexia, as shown in multiple animal models. In pancreatic islets of Langerhans isolated from carcinoma-bearing rats, insulin secretion is decreased in response to glucose stimulation, indicating impaired insulin sensitivity104. Despite decreased blood glucose levels in adenocarcinoma-bearing mice, insulin sensitivity is decreased, inducing insulin resistance66. Given the role of insulin in the maintenance of skeletal muscle, this evidence supports the involvement of insulin resistance in the development of tissue wasting in cancer cachexia patients.
Cardiac dysfunction, which occurs in human cancer patients and mouse cancer models, is closely associated with muscle wasting. Tumor-bearing rats show decreased heart weight and deterioration of the function of the heart, eventually leading to heart failure105. Increasing evidence indicates that cardiac atrophy is associated with growth inhibition by activating ACTRIIB, which is stimulated by TGF-β family ligands such as myostatin, activin, and growth/differentiation factor 11 (GDF11)106. Another study of heart alterations in cachexia found that cardiac oxygen consumption is increased in a mouse cancer model107. This increased oxygen consumption can be linked to increased energy expenditure by causing inefficient energy generation. Therefore, the elevated heart rate often seen in cancer patients could be a critical parameter of the risk of cancer death.
Concluding remarks
In this review, we discussed the key mechanisms of anorexia and tissue wasting induced by cancer cachexia. Despite more than 10 years of research into the mechanisms underlying cachexia, specific biomarkers for the diagnosis or treatment of this condition remain unavailable. Early detection of cancer cachexia syndrome increases treatment efficiency. Tumor-implanted rodent models are used for the discovery of the molecular mechanisms of cachexia. However, because the anorexia and wasting phenotypes of cachexia have different penetrance among cancer types, using different strains or different cancer models is critical to examine the variety of cachectic processes observed in cancer patients. In addition, because cachexia is a multiorgan syndrome that affects different tissues simultaneously, therapeutic strategies should be designed to target multiple organs.
Although cancer cachexia syndrome occurs independently from chemotherapy, there is evidence that chemotherapy can promote cachexia development, including anorexia21. However, the molecular mechanism underlying the effect of chemotherapy on inducing anorexia remains largely unknown. Therefore, it will be interesting to analyze whether known tumor-derived factors, such as INSL3 and IGFBP, are also involved in chemotherapy-induced cachexia.
References
Evans, W. J. et al. Cachexia: a new definition. Clin. Nutr. 27, 793–799 (2008).
Fearon, K. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12, 489–495 (2011).
Argiles, J. M., Busquets, S., Stemmler, B. & Lopez-Soriano, F. J. Cancer cachexia: understanding the molecular basis. Nat. Rev. Cancer 14, 754–762 (2014).
Tisdale, M. J. Mechanisms of cancer cachexia. Physiol. Rev. 89, 381–410 (2009).
Wagner, E. F. & Petruzzelli, M. Cancer metabolism: a waste of insulin interference. Nature 521, 430–431 (2015).
Argiles, J. M. et al. Consensus on cachexia definitions. J. Am. Med. Dir. Assoc. 11, 229–230 (2010).
Tisdale, M. J. Cancer anorexia and cachexia. Nutrition 17, 438–442 (2001).
Tazi, E. & Errihani, H. Treatment of cachexia in oncology. Indian J. Palliat. Care 16, 129–137 (2010).
Dewys, W. D. et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am. J. Med. 69, 491–497 (1980).
Teunissen, S. C. et al. Symptom prevalence in patients with incurable cancer: a systematic review. J. Pain. Symptom Manag. 34, 94–104 (2007).
Tisdale, M. J. Cachexia in cancer patients. Nat. Rev. Cancer 2, 862–871 (2002).
Warren, S. The immediate causes of death in cancer. Am. J. Med. Sci. 184, 610–615 (1932).
Inui, A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J. Clin. 52, 72–91 (2002).
Davis, M. P., Dreicer, R., Walsh, D., Lagman, R. & LeGrand, S. B. Appetite and cancer-associated anorexia: a review. J. Clin. Oncol. 22, 1510–1517 (2004).
Laviano, A. et al. Neural control of the anorexia-cachexia syndrome. Am. J. Physiol. Endocrinol. Metab. 295, E1000–E1008 (2008).
Tisdale, M. J. Catabolic mediators of cancer cachexia. Curr. Opin. Support Palliat. Care 2, 256–261 (2008).
Argiles, J. M., Busquets, S., Toledo, M. & Lopez-Soriano, F. J. The role of cytokines in cancer cachexia. Curr. Opin. Support Palliat. Care 3, 263–268 (2009).
Scheede-Bergdahl, C. et al. Is IL-6 the best pro-inflammatory biomarker of clinical outcomes of cancer cachexia? Clin. Nutr. 31, 85–88 (2012).
Droujinine, I. A. & Perrimon, N. Interorgan communication pathways in physiology: focus on drosophila. Annu. Rev. Genet. 50, 539–570 (2016).
Fearon, K., Arends, J. & Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 10, 90–99 (2013).
Ezeoke, C. C. & Morley, J. E. Pathophysiology of anorexia in the cancer cachexia syndrome. J. Cachexia Sarcopenia Muscle 6, 287–302 (2015).
Tsai, V. W. et al. Anorexia/cachexia of chronic diseases: a role for the TGF-beta family cytokine MIC-1/GDF15. J. Cachexia Sarcopenia Muscle 3, 239–243 (2012).
Johnen, H. et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat. Med. 13, 1333–1340 (2007).
Kir, S. et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 513, 100–104 (2014).
Kir, S. et al. PTH/PTHrP receptor mediates cachexia in models of kidney failure and cancer. Cell Metab. 23, 315–323 (2016).
Bozzetti, F. & Mariani, L. Defining and classifying cancer cachexia: a proposal by the SCRINIO Working Group. JPEN J. Parenter. Enter. Nutr. 33, 361–367 (2009).
Amitani, M., Asakawa, A., Amitani, H. & Inui, A. Control of food intake and muscle wasting in cachexia. Int. J. Biochem. Cell. Biol. 45, 2179–2185 (2013).
Morley, J. E., Thomas, D. R. & Wilson, M. M. Cachexia: pathophysiology and clinical relevance. Am. J. Clin. Nutr. 83, 735–743 (2006).
Imai, H., Soeda, H., Komine, K., Otsuka, K. & Shibata, H. Preliminary estimation of the prevalence of chemotherapy-induced dysgeusia in Japanese patients with cancer. BMC Palliat. Care 12, 38 (2013).
Noguchi, Y., Yoshikawa, T., Matsumoto, A., Svaninger, G. & Gelin, J. Are cytokines possible mediators of cancer cachexia? Surg. Today 26, 467–475 (1996).
Matthys, P. & Billiau, A. Cytokines and cachexia. Nutrition 13, 763–770 (1997).
Patra, S. K. & Arora, S. Integrative role of neuropeptides and cytokines in cancer anorexia-cachexia syndrome. Clin. Chim. Acta 413, 1025–1034 (2012).
Inui, A. Cytokines and sickness behavior: implications from knockout animal models. Trends Immunol. 22, 469–473 (2001).
Langhans, W. & Hrupka, B. Interleukins and tumor necrosis factor as inhibitors of food intake. Neuropeptides 33, 415–424 (1999).
Laviano, A. et al. Cracking the riddle of cancer anorexia. Nutrition 12, 706–710 (1996).
Martignoni, M. E., Kunze, P. & Friess, H. Cancer cachexia. Mol. Cancer 2, 36 (2003).
Siddiqui, R. A. & Williams, J. F. Tentative identification of the toxohormones of cancer cachexia: roles of vasopressin, prostaglandin E2 and cachectin-TNF. Biochem. Int. 20, 787–797 (1990).
Powrozek, T. et al. Relationship between TNF-alpha -1031T/C gene polymorphism, plasma level of TNF-alpha, and risk of cachexia in head and neck cancer patients. J. Cancer Res. Clin. Oncol. 144, 1423–1434 (2018).
Llovera, M. et al. Role of TNF receptor 1 in protein turnover during cancer cachexia using gene knockout mice. Mol. Cell. Endocrinol. 142, 183–189 (1998).
Yeom, E. et al. Tumour-derived Dilp8/INSL3 induces cancer anorexia by regulating feeding neuropeptides via Lgr3/8 in the brain. Nat. Cell. Biol. 23, 172–183 (2021).
Kwon, Y. et al. Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Developmental Cell 33, 36–46 (2015).
Figueroa-Clarevega, A. & Bilder, D. Malignant drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev. Cell 33, 47–55 (2015).
Petruzzelli, M. et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447 (2014).
Katheder, N. S. et al. Microenvironmental autophagy promotes tumour growth. Nature 541, 417–420 (2017).
Martinez-Sanchez, N. et al. Hypothalamic effects of thyroid hormones on metabolism. Best. Pract. Res. Clin. Endocrinol. Metab. 28, 703–712 (2014).
Schwartz, M. W., Woods, S. C., Porte, D., Seeley, R. J. & Baskin, D. G. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Woods, S. C., Seeley, R. J., Porte, D. Jr. & Schwartz, M. W. Signals that regulate food intake and energy homeostasis. Science 280, 1378–1383 (1998).
Woods, S. C., Schwartz, M. W., Baskin, D. G. & Seeley, R. J. Food intake and the regulation of body weight. Annu. Rev. Psychol. 51, 255–277 (2000).
Turton, M. D. et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379, 69–72 (1996).
Cowley, M. A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
Schwartz, M. W., Dallman, M. F. & Woods, S. C. Hypothalamic response to starvation: implications for the study of wasting disorders. Am. J. Physiol. 269, R949–R957 (1995).
Engineer, D. R. & Garcia, J. M. Leptin in anorexia and cachexia syndrome. Int. J. Pept. 2012, 287457 (2012).
Oh-I, S. et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443, 709–712 (2006).
Goebel, M., Stengel, A., Lambrecht, N. W. G., Wang, L. & Taché, Y. Nesfatin-1 immunoreactivity in rat brain and spinal cord autonomic nuclei. Neurosci. Lett. 452, 241–246 (2009).
Campos, C. A. et al. Cancer-induced anorexia and malaise are mediated by CGRP neurons in the parabrachial nucleus. Nat. Neurosci. 20, 934–942 (2017).
Emery, P. W., Edwards, R. H., Rennie, M. J., Souhami, R. L. & Halliday, D. Protein synthesis in muscle measured in vivo in cachectic patients with cancer. Br. Med. J. 289, 584–586 (1984).
Warnold, I., Lundholm, K. & Schersten, T. Energy balance and body composition in cancer patients. Cancer Res. 38, 1801–1807 (1978).
Chang, V. T., Xia, Q. & Kasimis, B. The functional assessment of anorexia/cachexia therapy (FAACT) appetite scale in veteran cancer patients. J. Support Oncol. 3, 377–382 (2005).
Martin, L. et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 31, 1539–1547 (2013).
Argiles, J. M., Fontes-Oliveira, C. C., Toledo, M., Lopez-Soriano, F. J. & Busquets, S. Cachexia: a problem of energetic inefficiency. J. Cachexia Sarcopenia Muscle 5, 279–286 (2014).
Constantinou, C. et al. Nuclear magnetic resonance in conjunction with functional genomics suggests mitochondrial dysfunction in a murine model of cancer cachexia. Int. J. Mol. Med. 27, 15–24 (2011).
Hardee, J. P., Montalvo, R. N. & Carson, J. A. Linking cancer cachexia-induced anabolic resistance to skeletal muscle oxidative metabolism. Oxid. Med. Cell Longev. 2017, 8018197 (2017).
Bonaldo, P. & Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model Mech. 6, 25–39 (2013).
Cohen, S., Nathan, J. A. & Goldberg, A. L. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 14, 58–74 (2015).
Penna, F. et al. Muscle atrophy in experimental cancer cachexia: is the IGF-1 signaling pathway involved? Int. J. Cancer 127, 1706–1717 (2010).
Asp, M. L., Tian, M., Wendel, A. A. & Belury, M. A. Evidence for the contribution of insulin resistance to the development of cachexia in tumor-bearing mice. Int. J. Cancer 126, 756–763 (2010).
Costelli, P. et al. IGF-1 is downregulated in experimental cancer cachexia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R674–R683 (2006).
Garcia, J. M., Friend, J. & Allen, S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study. Support Care Cancer 21, 129–137 (2013).
Garcia, J. M. et al. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J. Clin. Endocrinol. Metab. 90, 2920–2926 (2005).
Rofe, A. M., Bourgeois, C. S., Coyle, P., Taylor, A. & Abdi, E. A. Altered insulin response to glucose in weight-losing cancer patients. Anticancer Res. 14, 647–650 (1994).
Mantovani, G. et al. Serum levels of leptin and proinflammatory cytokines in patients with advanced-stage cancer at different sites. J. Mol. Med. 78, 554–561 (2000).
Acharyya, S. et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J. Clin. Invest. 114, 370–378 (2004).
Baltgalvis, K. A. et al. Interleukin-6 and cachexia in ApcMin/+ mice. Am. Physiol. Regul. Integr. Comp. Physiol. 294, R393–R401 (2008).
Mantovani, G. et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 15, 200–211 (2010).
Cannon, T. et al. Immunocompetent murine model of cancer cachexia for head and neck squamous cell carcinoma. Head. Neck 30, 320–326 (2008).
Mi, L. et al. Bacterial translocation contributes to cachexia from locally advanced gastric cancer. Hepatogastroenterology 59, 2348–2351 (2012).
Glass, D. J. Signaling pathways perturbing muscle mass. Curr. Opin. Clin. Nutr. Metab. Care 13, 225–229 (2010).
Cai, D. et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119, 285–298 (2004).
Moore-Carrasco, R. et al. The AP-1/NF-kappaB double inhibitor SP100030 can revert muscle wasting during experimental cancer cachexia. Int. J. Oncol. 30, 1239–1245 (2007).
Huang, X.-y et al. Pancreatic cancer cell-derived IGFBP-3 contributes to muscle wasting. J. Exp. Clin. Cancer Res. 35, 46 (2016).
Tsai, V. W., Lin, S., Brown, D. A., Salis, A. & Breit, S. N. Anorexia-cachexia and obesity treatment may be two sides of the same coin: role of the TGF-b superfamily cytokine MIC-1/GDF15. Int. J. Obes. 40, 193–197 (2016).
Coll, A. P. et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 578, 444–448 (2020).
Chang, J. Y. et al. The role of growth differentiation factor 15 in energy metabolism. Diabetes Metab. J. 44, 363–371 (2020).
Day, E. A. et al. Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss. Nat. Metab. 1, 1202–1208 (2019).
Suriben, R. et al. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat. Med. 26, 1264–1270 (2020).
Argiles, J. M., Lopez-Soriano, J., Almendro, V., Busquets, S. & Lopez-Soriano, F. J. Cross-talk between skeletal muscle and adipose tissue: a link with obesity? Med. Res. Rev. 25, 49–65 (2005).
Torti, F. M., Dieckmann, B., Beutler, B., Cerami, A. & Ringold, G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science 229, 867–869 (1985).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Wang, G., Zhang, H. & Lyden, D. Tumour-regulated anorexia preceding cachexia. Nat. Cell. Biol. 23, 111–113 (2021).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Herremans, K. M., Riner, A. N., Cameron, M. E. & Trevino, J. G. The microbiota and cancer cachexia. Int. J. Mol. Sci. 20, 6267 (2019).
Ziemons, J., Smidt, M. L., Damink, S. O. & Rensen, S. S. Gut microbiota and metabolic aspects of cancer cachexia. Best. Pract. Res. Clin. Endocrinol. Metab. 35, 101508 (2021).
Bindels, L. B. & Delzenne, N. M. Muscle wasting: the gut microbiota as a new therapeutic target? Int. J. Biochem. Cell Biol. 45, 2186–2190 (2013).
Watanabe, M. et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489 (2006).
Morley, J. E., Farr, S. A., Sell, R. L., Hileman, S. M. & Banks, W. A. Nitric oxide is a central component in neuropeptide regulation of appetite. Peptides 32, 776–780 (2011).
Otto, B. et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur. J. Endocrinol. 145, 669–673 (2001).
DeBoer, M. D. et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology 148, 3004–3012 (2007).
Sheriff, S. et al. Des-acyl ghrelin exhibits pro-anabolic and anti-catabolic effects on C2C12 myotubes exposed to cytokines and reduces burn-induced muscle proteolysis in rats. Mol. Cell Endocrinol. 351, 286–295 (2012).
Neary, N. M. et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J. Clin. Endocrinol. Metab. 89, 2832–2836 (2004).
Hiura, Y. et al. Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients: a prospective, randomized, placebo-controlled phase 2 study. Cancer 118, 4785–4794 (2012).
Honors, M. A. & Kinzig, K. P. Chronic exendin-4 treatment prevents the development of cancer cachexia symptoms in male rats bearing the Yoshida sarcoma. Horm. Cancer 5, 33–41 (2014).
Borner, T., Liberini, C. G., Lutz, T. A. & Riediger, T. Brainstem GLP-1 signalling contributes to cancer anorexia-cachexia syndrome in the rat. Neuropharmacology 131, 282–290 (2018).
Tayek, J. A. A review of cancer cachexia and abnormal glucose metabolism in humans with cancer. J. Am. Coll. Nutr. 11, 445–456 (1992).
Fernandes, L. C., Machado, U. F., Nogueira, C. R., Carpinelli, A. R. & Curi, R. Insulin secretion in Walker 256 tumor cachexia. Am. J. Physiol. 258, E1033–E1036 (1990).
Olivan, M. et al. Theophylline is able to partially revert cachexia in tumour-bearing rats. Nutr. Metab. (Lond.) 9, 76 (2012).
Zhou, X. et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142, 531–543 (2010).
Drott, C. & Lundholm, K. Glucose uptake and amino acid metabolism in perfused hearts from tumor-bearing rats. J. Surg. Res. 49, 62–68 (1990).
Acknowledgements
This work is supported by grants from the KRIBB Research Initiative Program and National Research Foundation of Korea (2019R1A2C2004149).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yeom, E., Yu, K. Understanding the molecular basis of anorexia and tissue wasting in cancer cachexia. Exp Mol Med 54, 426–432 (2022). https://doi.org/10.1038/s12276-022-00752-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-022-00752-w
This article is cited by
-
The effects of synbiotics supplementation on reducing chemotherapy-induced side effects in women with breast cancer: a randomized placebo-controlled double-blind clinical trial
BMC Complementary Medicine and Therapies (2023)
-
Cancer-associated cachexia — understanding the tumour macroenvironment and microenvironment to improve management
Nature Reviews Clinical Oncology (2023)
-
Advances in pharmacotherapies in cancer-related cachexia*
Oncology and Translational Medicine (2023)