Abstract
Neurons in the central nervous system (CNS) communicate with peripheral organs largely via the autonomic nervous system (ANS). Through such communications, the sympathetic and parasympathetic efferent divisions of the ANS may affect thermogenesis and blood glucose levels. In contrast, peripheral organs send feedback to the CNS via hormones and autonomic afferent nerves. These humoral and neural feedbacks, as well as neural commands from higher brain centers directly or indirectly shape the metabolic function of autonomic neurons. Notably, recent developments in mouse genetics have enabled more detailed studies of ANS neurons and circuits, which have helped elucidate autonomic control of metabolism. Here, we will summarize the functional organization of the ANS and discuss recent updates on the roles of neural and humoral factors in the regulation of energy balance and glucose homeostasis by the ANS.
Similar content being viewed by others
Introduction
The autonomic nervous system (ANS) serves as a key structure to mediate unconscious regulation of bodily function by the central nervous system (CNS). In particular, the hypothalamus utilizes the sympathetic and parasympathetic divisions of the ANS to innervate peripheral organs and to control the metabolic function of our body. For instance, anorexigenic (appetite-suppressing) pro-opiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus (ARH) activate sympathetic preganglionic neurons in the spinal cord, which in turn increases thermogenesis in brown adipose tissue (BAT)1,2. In addition, POMC neurons in the ARH reportedly regulate parasympathetic preganglionic neurons in the brainstem, which decreases insulin secretion from pancreatic β-cells3,4. In these examples, the activity of autonomic neurons is affected by α-melanocyte-stimulating hormone (α-MSH), a neuropeptide released from POMC neurons in the ARH, which acts on the anorectic melanocortin-4 receptor (MC4R). As such, autonomic neurons are influenced by neuropeptides and neurotransmitters5,6,7,8. In addition, peripheral hormones such as insulin and glucagon-like peptide-1 (GLP-1) were demonstrated to regulate the activity of autonomic neurons9,10,11. Thus, it appears that neurons of the ANS translate neural and humoral signals into commands that directly regulate peripheral organs and metabolic function. Together, these results demonstrate how the efferent (motor) divisions of the ANS regulate metabolism.
However, recent evidence has demonstrated that parasympathetic or vagal afferent (sensory) fibers inform the CNS of food in the gut. For instance, it was demonstrated that ingestion of food causes mechanical stretch of the stomach or intestinal walls, which is relayed by vagal sensory neurons to the CNS and stimulates anorexigenic neurons or inhibits orexigenic (appetite-promoting) neurons to stop feeding12,13. In addition, recent studies have suggested that the gut microbiome stimulates vagal sensory neurons to affect many facets of metabolic function14,15. Therefore, both efferent and afferent divisions of the ANS can regulate energy balance and glucose homeostasis.
Here, we discuss key structures of the ANS, focusing on the role of ANS neurons in the regulation of feeding and metabolism. We also summarize how neural, humoral, and other factors modulate efferent and afferent divisions of the ANS and their metabolic function.
Metabolic function of the autonomic nervous system
Role of autonomic motor function
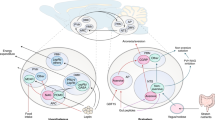
The parasympathetic and sympathetic nervous systems represent the motor (efferent) part of the ANS, which innervates internal organs and regulates their function16 (Fig. 1). Neurons of the ANS, parasympathetic or sympathetic, are categorized into preganglionic and postganglionic neurons; the cell bodies of preganglionic neurons are located within the CNS (brainstem and spinal cord), whereas those of postganglionic neurons comprise autonomic ganglia found in body cavities or peripheral target organs. In particular, the parasympathetic and sympathetic divisions are anatomically segregated. The cell bodies of parasympathetic preganglionic neurons projecting to the internal organs are located in the brainstem, where they make up the dorsal motor nucleus of the vagus (DMV) and the nucleus ambiguus. Notably, although the intermediolateral column (IML) of the sacral spinal cord is conventionally thought to be a part of the parasympathetic division, a recent study suggested that sacral autonomic outflow may be sympathetic17 since the developmental and transcriptional traits of sacral autonomic neurons are similar to those of sympathetic preganglionic neurons rather than parasympathetic preganglionic neurons. The parasympathetic postganglionic neurons are located in the target organs and compose synapses with preganglionic axon terminals8,18. However, the preganglionic neurons of the sympathetic division are found in the IML of the thoracic to the upper lumbar spinal cord19,20. The sympathetic ganglia are typically located outside of the target organs, where the sympathetic postganglionic neurons receive synaptic inputs from the sympathetic preganglionic neurons. For instance, the sympathetic postganglionic neurons innervating the BAT are located in the stellate ganglia21, whereas those innervating abdominal organs such as the digestive tract, pancreas, liver, and some white adipose tissues (WAT) are located in the celiac ganglia22,23,24.
The parasympathetic preganglionic neurons (blue dots) are located in the DMV of the brainstem, while the sympathetic preganglionic neurons (red dots) are located in the IML of the thoracic and upper lumbar spinal cord. The parasympathetic preganglionic neurons located in the nucleus ambiguus and the IML of the sacral spinal cord are not shown. The parasympathetic postganglionic neurons (blue dots) are located in the peripheral target organs, while the sympathetic postganglionic neurons (red dots) are located in the sympathetic ganglia within the abdominal cavity. The parasympathetic efferent (blue lines) and sympathetic efferent (red lines) fibers innervate peripheral organs that regulate metabolism, including BAT, pancreas, liver, and WAT. Note that BAT and WAT receive only sympathetic innervation, whereas the pancreas and liver are innervated by both parasympathetic and sympathetic efferent nerves. The parasympathetic afferent fibers (purple lines) have cell bodies (purple dots) in the NG, which send peripheral information to neurons of the NTS (black dots) and AP (neurons not shown). See the text for abbreviations.
Both preganglionic and postganglionic neurons of the parasympathetic division release acetylcholine (ACh) from their terminals. Sympathetic preganglionic neurons also release ACh, but sympathetic postganglionic neurons are unique in that they use norepinephrine (NE) as the major neurotransmitter. Therefore, choline acetyltransferase (ChAT), which is a key enzyme for the synthesis of ACh, can serve as a useful chemical marker for cholinergic autonomic neurons. Using mice with Cre recombinase activity under the control of the ChAT promoter (ChAT-cre mice), researchers manipulated gene expression in a cholinergic neuron-specific manner to identify the role of specific molecules expressed by autonomic neurons25,26. Paired-like homeobox 2b (Phox2b) is a transcription factor that is known to mediate the development of the parasympathetic nervous system. Thus, scientists have used Phox2b-cre mice to manipulate neurons of the parasympathetic division of the ANS26. No mouse model is currently available to selectively label sympathetic neurons.
The ANS innervates multiple organs that regulate metabolism; the pancreas and the liver receive both sympathetic and parasympathetic innervation, whereas adipose tissues receive only sympathetic innervation23 (Fig. 1). The parasympathetic nervous system promotes insulin secretion, as evidenced by the impaired insulin secretion observed in vagotomized rats27. However, the sympathetic nervous system stimulates glucagon secretion28. A recent study reported that parasympathetic and sympathetic neuronal signaling regulates β-cell proliferation29. The parasympathetic and sympathetic nervous systems also affect liver function18. The parasympathetic nervous system inhibits the gluconeogenic pathway in the liver30, which may contribute to lower blood glucose levels. In contrast, the sympathetic nervous system stimulates gluconeogenic and glycogenolytic pathways in the liver to elevate the blood glucose level31. The ANS also has an impact on hepatic lipid metabolism32. The sympathetic nervous system enhances very-low-density lipoprotein (VLDL) synthesis and triglyceride (TG) secretion; impaired sympathetic function has been linked to the pathogenesis of nonalcoholic fatty liver disease (NAFLD)33,34. In addition, the sympathetic nervous system is an important regulator of WAT function, as evidenced by lipolysis induced by NE released from the synaptic end of sympathetic postganglionic neurons35. A recent study reported that sympathetic stimulations even lead to the browning of WAT36. The sympathetic nervous system also stimulates thermogenesis in the BAT of rodents37. In human subjects, BAT was originally known to exist only in infants, but a recent study reported that adults also have functional BAT38. These results further highlight the importance of the sympathetic nervous system as a potential target for the treatment of obesity.
Role of autonomic sensory function
The sympathetic sensory fibers are intermingled with somatic sensory fibers and thus are not readily dissected anatomically39. However, the parasympathetic nervous system has afferent fibers dedicated to sensory function. The vagal sensory neurons are bipolar neurons that project from peripheral organs to the brain stem. The somata of the vagal sensory neurons are located in the nodose ganglia (NG) (Fig. 1). The stimulation of vagal sensory (or NG) neurons was reported to result in the suppression of feeding40. Interestingly, experimental evidence from multiple recent studies suggested that NG neurons are highly heterogeneous. A recent study using novel sequencing techniques revealed that NG neurons have a highly localized and compartmentalized structure; the peripheral axons of calcitonin gene-related peptide (CGRP)-expressing NG neurons form a structure called the mucosal endings in the gut, while those of oxytocin receptor (Oxtr)-expressing NG neurons form a structure called the intestinal intraganglionic laminar endings12. Notably, optogenetic and chemogenetic activation of Oxtr-expressing NG neurons inhibited food intake, while stimulation of CGRP-expressing NG neurons had no effects. These results suggested that stimulation of a specific subpopulation of NG neurons is sufficient to inhibit feeding. Additionally, it is worthwhile to note that the right and left NGs were reported to be anatomically and functionally distinct41. Most neurons in the right NG innervate the nucleus tractus solitarius (NTS), whereas most neurons in the left NG innervate the area postrema (AP). Optogenetic stimulation of axon terminals of NG neurons, right or left, induced a significant decrease in chow intake. However, only stimulation of the right NG neuronal axon terminal resulted in place preference. These results suggested that the activity of the right NG to the NTS circuit is sufficient to induce motivated behavior. The right NG to NTS circuit was found to be connected to the dorsolateral aspect of the parabrachial nucleus (PBN), dopaminergic neurons in the midbrain, and striatum. Finally, a subpopulation of NG neurons was reported to be glucose-sensing neurons42. These glucose-sensing neurons may also suppress feeding in vivo, although this hypothesis needs to be confirmed by direct experimental evidence. The vagal sensory neurons, especially those responsible for chemical sensing, can be labeled using Nav1.8-cre mice43. This mouse model was used to identify the metabolic function of molecules expressed by vagal sensory neurons44,45.
As mentioned previously, vagal sensory information is transferred to neurons in the NTS8 and AP41. The NTS, like the NG, contains many types of neurons that are also functionally heterogeneous. NTS neurons that express either cholecystokinin (CCK) or dopamine β-hydroxylase are activated by food intake, and these neurons provide excitatory input to anorexigenic CGRP-expressing PBN neurons46. CCK-expressing NTS neurons were also shown to project to the paraventricular nucleus of the hypothalamus (PVH), which is a major satiety center47. In addition, many POMC neurons in the NTS express CCK and the serotonin 2C receptor, which innervate forebrain structures to induce anorexia47,48. However, NTS neurons are not always anorexigenic. Tyrosine hydroxylase (TH)-expressing NTS neurons reportedly use NE as a neurotransmitter to innervate orexigenic agouti-related peptide (AgRP) neurons within the ARH, where the release of NE directly excites AgRP neurons49. Inhibition of these neurons suppressed food intake when the mice were under glucoprivic hunger, which was induced by 2-deoxyglucose. Interestingly, inhibition of TH-expressing NTS neurons failed to suppress food intake when the mice were subjected to food deprivation.
The PBN receives ascending sensory inputs from the NTS and AP12. Recently, a study demonstrated that prodynorphin (Pdyn)-expressing PBN neurons receive information regarding mechanical stretching via the NTS, which mediates anorexia and negative valence13. These results suggested that mechanical stretch induced by food in the stomach is sensed by local vagal afferent neurons and transmitted to the NTS and PBN to suppress feeding. Previous studies have suggested that vagus nerve stimulation (VNS), which was originally approved for treatment-resistant epilepsy50 and depression51, is also effective in reducing food intake52,53,54. VNS is now approved by the Food and Drug Admistration (FDA) for the treatment of obesity. Since VNS affects both the afferent and efferent arms of the vagus nerve through the application of electricity via patches attached to the skin, it is not clear how VNS can reduce food intake. Nonetheless, we envision that the central pathways involving neurons of the NTS, PBN, and possibly the hypothalamus are responsible for the anorexigenic effects.
Neural control of autonomic function
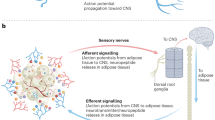
Parasympathetic preganglionic neurons were previously shown to receive direct and indirect neuronal projections from several nuclei of the hypothalamus55 (Fig. 2). Notably, axon terminals that innervate the parasympathetic preganglionic neurons of the DMV are frequently found in the NTS56. It is reasonable to assume that neurons in the NTS receive those inputs and relay the information to the parasympathetic preganglionic neurons of the DMV. Indeed, neurons in the NTS directly innervate neurons in the DMV via either GABAergic or glutamatergic fibers57,58,59, and the NTS to DMV GABAergic circuit has been shown to control glucose homeostasis59. Alternatively, axon terminals may synapse onto the dendrites of parasympathetic preganglionic neurons that extend into the NTS to directly receive hypothalamic inputs. For example, NPY-expressing DMH neurons monosynaptically innervate Y1 receptor (Y1R)-expressing DMV neurons60. This neural circuit was not responsible for the regulation of feeding or body weight but was involved in the maintenance of glucose homeostasis by increasing hepatic glucose production (HGP). In contrast, relatively limited data are available on the neural control of sympathetic preganglionic neurons, which is probably due to the technical difficulty of studying neural circuits in the spinal cord. It was previously shown that neurons in the hypothalamus and brainstem innervate the IML5,61, but the functional significance of these connections remains to be determined.
The parasympathetic preganglionic neurons of the DMV (lower left) receive neural input from neurons of the NTS (upper left) and the hypothalamic nuclei (center). The sympathetic preganglionic neurons of the IML (lower right) receive neural input from neurons of the brainstem (upper right) and ARH POMC neurons (lower center). Only selective major innervations are shown for clarity. See the text for abbreviations.
One of the well-characterized inputs to autonomic preganglionic neurons within the DMV and IML originates from arcuate POMC neurons5,62. As mentioned previously, POMC neurons release α-MSH, which is a full agonist of MC4R63. Both parasympathetic and sympathetic preganglionic neurons express functional MC4Rs61. MC4Rs expressed by sympathetic preganglionic neurons were shown to increase BAT thermogenesis and blood pressure but decrease HGP26. In addition, MC4Rs expressed by parasympathetic preganglionic neurons were suggested to decrease insulin secretion26. Interestingly, patch-clamp studies demonstrated that MC4R agonists depolarize (or activate) sympathetic preganglionic neurons while hyperpolarizing (or inhibiting) parasympathetic preganglionic neurons25, which suggests that stimulations of MC4Rs increase sympathetic tone. These results at least in part explain the autonomic phenotypes observed in MC4R-deficient mice and human patients with MC4R mutations, including decreased thermogenesis, hyperinsulinemia, and resistance to obesity-induced hypertension. However, it remains unclear how MC4Rs normalize (or reduce) HGP by increasing sympathetic activity. In addition, there is currently no evidence that the activity of autonomic preganglionic neurons is modulated by α-MSH release in vivo. These remaining issues need to be resolved in future investigations. However, little is known regarding the regulation of the ANS by other neuropeptides released from hypothalamic neurons. One example is orexin (or hypocretin), which is a neuropeptide synthesized by a discrete set of neurons within the lateral hypothalamic area (LHA) to control feeding behavior and arousal64. It was previously demonstrated that LHA orexin neurons project to gut-projecting DMV neurons that putatively express orexin receptor 1/2 (OR1/2) to control gastric function65,66.
Neurons that directly innervate sympathetic preganglionic neurons in the IML are called sympathetic premotor neurons67. Sympathetic premotor neurons are typically found in the rostral medulla. Sympathetic premotor neurons within the rostral ventrolateral medulla (RVLM) are known to control cardiovascular functions68. In particular, MC4R-expressing RVLM neurons innervate the IML neurons that project to the lung69. Unlike the RVLM, those in the rostral medullary raphe regions reportedly regulate thermogenesis70,71. In particular, sympathetic premotor neurons located within the rostral part of the raphe pallidus (RPa) and raphe magnus were suggested to be involved in thermoregulation. A previous study demonstrated that optogenetic stimulation of cholinergic neurons decreased BAT thermogenesis via muscarinic M2 receptors expressed by RPa serotonergic neurons72. In another study, serotonergic neurons located in the dorsal raphe nuclei (DRN) projected to the RPa and functionally modulated BAT energy expenditure6. Given the role of DRN serotonergic neurons in regulating thermogenesis and locomotor activity73,74, the DRN→RPa circuit may represent an effector system to excite the sympathetic nervous system.
Humoral control of autonomic function
Parasympathetic preganglionic neurons are also influenced by peripheral hormones, which may enter the CNS via circumventricular organs (CVOs) where the blood-brain barrier is not very tight75,76. Thus, peripheral hormones may have access to neurons within the NTS and DMV via the AP, which has characteristics of CVO. In the case of sympathetic preganglionic neurons, there is no nearby structure that can serve as a CVO. Therefore, sympathetic preganglionic neurons have only limited access to peripheral hormones, which may be why there are currently no data regarding humoral regulation of sympathetic preganglionic neurons. Most results regarding the role of hormones in the regulation of autonomic neurons were obtained from studies using in vivo conditional knockout mouse models and ex vivo electrophysiology experiments.
Leptin and leptin receptors (LepRs) were first reported in the 1990s77,78. Leptin is a unique fat cell-derived hormone, and many scientists have studied this hormone in the context of feeding and metabolism. Indeed, mice and human subjects lacking leptin or LepRs develop obesity, which is accompanied by decreased energy expenditure and increased food intake79,80. In particular, the abundant expression of LepRs by central neurons has prompted researchers to study the role of leptin in the CNS81,82,83,84. While deletions of LepRs in a single population of neurons failed to reproduce the obesity phenotypes observed in whole-body knockout mice85,86, Lowell and colleagues found that LepR deficiency in GABAergic neurons produces obesity87. These results highlighted the role of GABAergic neurons in mediating the metabolic effects of leptin, but the anatomical location of the responsible GABAergic neurons is still unknown. In the ANS, LepR deficiency in Phox2b neurons did not result in a body weight phenotype, although both food intake and energy expenditure were increased88. These results suggest that LepRs expressed by parasympathetic neurons cause changes in either food intake or energy expenditure, which is readily compensated. Multiple studies from independent groups reported that leptin applications inhibit the activity of DMV neurons via phosphoinositide 3-kinase (PI3K)-dependent activation of ATP-sensitive potassium (KATP) channels89,90 (Table 1). However, it is currently not clear whether leptin-induced inhibition of DMV neurons causes changes in food intake or energy expenditure. Insulin is another peripheral hormone that controls parasympathetic neurons. It was reported that insulin also inhibits DMV neurons via PI3K-dependent activation of KATP channels9. Interestingly, parasympathetic preganglionic neurons stimulate the secretion of insulin from pancreatic β-cells3. Therefore, the suppression of DMV neuronal activity by insulin may represent a negative feedback loop. However, it remains to be determined whether such homeostatic regulation exists in animals.
In addition to leptin and insulin, hormones released from gut endocrine cells were demonstrated to affect autonomic function. For instance, while GLP-1 is secreted largely from gut endocrine cells, GLP-1 has its cognate receptor (GLP-1 receptor or GLP-1R) throughout the brain. In particular, it was shown that DMV neurons also express GLP-1R91 and that pancreas-projecting GLP-1R-expressing neurons are excited by the application of GLP-1 directly via the closure of putative K+ conductance and indirectly via GABA-activated Cl- conductance92. The excitation of DMV neurons and the increased parasympathetic tone may contribute to the well-known insulinotropic effects of GLP-1. Interestingly, by using Phox2b-cre-specific GLP-1R knockout mice, researchers reported that the conditional knockout mice show decreased food intake, glucose tolerance, and accelerated gastric emptying10. However, knockdown of GLP-1R in neurons of the NTS reportedly resulted in increased food intake in the dark cycle11.
Another example is CCK, which was originally identified as a gut modulator acting on vagal afferent fibers93. CCK is released from duodenal endocrine cells in isoforms such as CCK58, CCK22, and CCK8. Neurons of the DMV express CCK receptor 1 (CCK1R), and CCK8 generates inward currents by decreasing putative K+ conductance94. Later, it was shown that CCK8 depolarizes the membrane potential of DMV neurons directly by decreasing K+ currents and indirectly by decreasing the frequency of spontaneous excitatory postsynaptic currents (sEPSCs)95. CCK1R is also expressed by neurons of the NTS96, and it was shown that in the postprandial period, c-Fos activity and phosphorylated extracellular signal-regulated kinase 1/2 (pERK1/2) levels are increased in CCK1R-expressing NTS neurons97,98. Moreover, CCK was demonstrated to activate NTS POMC neurons, which may play a role in generating satiety99,100. Therefore, both GLP-1 and CCK appear to affect appetite and metabolism by acting on both motor and sensory parts of the parasympathetic nervous system.
Concluding remarks
The ANS has a major role in the control of energy balance and glucose homeostasis; sympathetic activity increases thermogenesis and hepatic gluconeogenesis, parasympathetic activity promotes insulin secretion, and vagal sensory neurons signal fullness. Therefore, it is essential to determine the mechanisms in autonomic neurons and the circuits to obtain a comprehensive understanding of whole-body metabolism in health and disease. “Conventional” autonomic neuroscience utilizes histology and electrophysiology as the major tools. Currently, findings obtained from these experiments are continuously being corroborated with findings using fine genetic tools, including mouse genetics, optogenetics, and chemogenetics. As a result, we now have more detailed information regarding autonomic control of appetite and metabolism.
Given that neurons of the ANS not only regulate appetite and metabolism but also control a variety of key homeostatic functions, such as cardiac activity and breathing, it is very likely that other functions, including circulation and respiration, influence metabolism and that the ANS serves as an important mediator between these functions. For example, we need more blood and oxygen to metabolize nutrients after each meal, and the ANS likely performs fine-tuning of these homeostatic functions. Fortunately, many advancements have recently been made in other fields of neuroscience, and the cutting-edge techniques used therein could be applied to study autonomic function. However, autonomic circuitry is not as straightforward as central neural circuits since the former includes the interface between peripheral organs and peripheral/central neurons. Therefore, we need to focus on autonomic neuroscience and develop more advanced methods to investigate autonomic function and circuits. We believe that these efforts will help to gain novel insight into the autonomic function and to result in additional therapeutic options for obesity and metabolic diseases.
References
Swanson, L. W. & Kuypers, H. G. J. M. A direct projection from the ventromedial nucleus and retrochiasmatic area of the hypothalamus to the medulla and spinal cord of the rat. Neurosci. Lett. 17, 307–312 (1980).
Elias, C. F. et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21, 1375–1385 (1998).
Ionescu, E., Rohner-Jeanrenaud, F., Berthoud, H. R. & Jeanrenaud, B. Increases in plasma insulin levels in response to electrical stimulation of the dorsal motor nucleus of the vagus nerve. Endocrinology 112, 904–910 (1983).
Kwon, E. et al. Optogenetic stimulation of the liver-projecting melanocortinergic pathway promotes hepatic glucose production. Nat. Commun. 11, 6295 (2020).
Sohn, J.-W. W., Elmquist, J. K. & Williams, K. W. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 36, 504–512 (2013).
Schneeberger, M. et al. Regulation of energy expenditure by brainstem GABA neurons. Cell 178, 672–685.e12 (2019).
Li, L. et al. Knockdown of neuropeptide y in the dorsomedial hypothalamus promotes hepatic insulin sensitivity in male rats. Endocrinology 157, 4842–4852 (2016).
Travagli, R. A., Hermann, G. E., Browning, K. N. & Rogers, R. C. Brainstem circuits regulating gastric function. Annu. Rev. Physiol. 68, 279–305 (2006).
Blake, C. B. & Smith, B. N. Insulin reduces excitation in gastric-related neurons of the dorsal motor nucleus of the vagus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R807–R814 (2012).
Varin, E. M. et al. Distinct neural sites of GLP-1R expression mediate physiological versus pharmacological control of incretin action. Cell Rep. 27, 3371–3384.e3 (2019).
Alhadeff, A. L. et al. Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is required for food intake. Control. Neuropsychoprarmacol. 42, 1471–1479 (2017).
Bai, L. et al. Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129–1143.e23 (2019).
Kim, D. Y. et al. A neural circuit mechanism for mechanosensory feedback control of ingestion. Nature 580, 376–380 (2020).
Bonaz, B., Bazin, T. & Pellissier, S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 12, 49 (2018).
Pradhananga, S., Tashtush, A. A., Allen-Vercoe, E., Petrof, E. O. & Lomax, A. E. Protease-dependent excitation of nodose ganglion neurons by commensal gut bacteria. J. Physiol. 598, 2137–2151 (2020).
Gibbons, C. H. In Handbook of Clinical Neurology Vol. 160, 407–418 (Elsevier B.V., 2019).
Espinosa-Medina, I. et al. The sacral autonomic outflow is sympathetic. Science 354, 893–897 (2016).
Yi, C.-X. X., la Fleur, S. E., Fliers, E. & Kalsbeek, A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim. Biophys. Acta 1802, 416–431 (2010).
Appel, N. M. & Elde, R. P. The intermediolateral cell column of the thoracic spinal cord is comprised of target-specific subnuclei: evidence from retrograde transport studies and immunohistochemistry. J. Neurosci. 8, 1767–1775 (1988).
Zhou, S. Y. & Gilbey, M. P. Respiratory‐related activity of lower thoracic and upper lumbar sympathetic preganglionic neurones in the rat. J. Physiol. 451, 631–642 (1992).
François, M. et al. Sympathetic innervation of the interscapular brown adipose tissue in mouse. Ann. N. Y. Acad. Sci. 1454, 3–13 (2019).
Li, W., Yu, G., Liu, Y. & Sha, L. Intrapancreatic ganglia and neural regulation of pancreatic endocrine secretion. Front. Neurosci. 13, 21 (2019).
Bartness, T. J., Song, C. K., Shi, H., Bowers, R. R. & Foster, M. T. Brain–adipose tissue cross talk. Proc. Nutr. Soc. 64, 53–64 (2005).
Li, M., Galligan, J., Wang, D. & Fink, G. The effects of celiac ganglionectomy on sympathetic innervation to the splanchnic organs in the rat. Auton. Neurosci. Basic Clin. 154, 66–73 (2010).
Sohn, J. W. et al. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell 152, 612–619 (2013).
Rossi, J. et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 13, 195–204 (2011).
Yamatani, K. et al. Impaired vagus nerve-mediated control of insulin secretion in Wistar fatty rats. Metabolism 47, 1167–1173 (1998).
Ahren, B., Veith, R. C. & Taborsky, G. J. Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1) effects on basal release of insulin and glucagon. Endocrinology 121, 323–331 (1987).
Moullé, V. S. et al. The autonomic nervous system regulates pancreatic β-cell proliferation in adult male rats. Am. J. Physiol. Endocrinol. Metab. 317, E234–E243 (2019).
Pocal, A. et al. Hypothalamic KATP channels control hepatic glucose production. Nature 434, 1026–1031 (2005).
Chan, T. M. & Exton, J. H. Studies on α-adrenergic activation of hepatic glucose output. Studies on α-adrenergic inhibition of hepatic pyruvate kinase and activation of gluconeogenesis. J. Biol. Chem. 253, 6393–6400 (1978).
Bruinstroop, E., Fliers, E. & Kalsbeek, A. Hypothalamic control of hepatic lipid metabolism via the autonomic nervous system. Best. Pract. Res. Clin. Endocrinol. Metab. 28, 673–684 (2014).
Tavares, F. L. & Seelaender, M. C. L. Hepatic denervation impairs the assembly and secretion of VLDL-TAG. Cell Biochem. Funct. 26, 557–565 (2008).
Amir, M., Yu, M., He, P. & Srinivasan, S. Hepatic autonomic nervous system and neurotrophic factors regulate the pathogenesis and progression of non-alcoholic fatty liver disease. Front. Med. 7, 62 (2020).
Bartness, T. J., Liu, Y., Shrestha, Y. B. & Ryu, V. Neural innervation of white adipose tissue and the control of lipolysis. Front. Neuroendocrinol 35, 473–493 (2014).
Cao, Q., Jing, J., Cui, X., Shi, H. & Xue, B. Sympathetic nerve innervation is required for beigeing in white fat. Physiol. Rep. 7, e14031 (2019).
Desautels, M. & Dulos, R. A. Effects of neonatal sympathectomy on brown fat development and susceptibility to high fat diet induced obesity in mice. Can. J. Physiol. Pharmacol. 69, 1868–1874 (1991).
Virtanen, K. A. The rediscovery of BAT in adult humans using imaging. Best. Pract. Res. Clin. Endocrinol. Metab. 30, 471–477 (2016).
Nascimento, A. I., Mar, F. M. & Sousa, M. M. The intriguing nature of dorsal root ganglion neurons: Linking structure with polarity and function. Prog. Neurobiol. 168, 86–103 (2018).
Beutler, L. R. et al. Dynamics of gut-brain communication underlying hunger. Neuron 96, 461–475 (2017).
Han, W. et al. A neural circuit for gut-induced reward. Cell 175, 665–678.e23 (2018).
Grabauskas, G., Zhou, S. Y., Lu, Y., Song, I. & Owyang, C. Essential elements for glucosensing by gastric vagal afferents: immunocytochemistry and electrophysiology studies in the rat. Endocrinology 154, 296–307 (2013).
Gautron, L. et al. Genetic tracing of Nav1.8-expressing vagal afferents in the mouse. J. Comp. Neurol. 519, 3085–3101 (2011).
Udit, S. et al. Nav1.8 neurons are involved in limiting acute phase responses to dietary fat. Mol. Metab. 6, 1081–1091 (2017).
de Lartigue, G., Ronveaux, C. C. & Raybould, H. E. Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol. Metab. 3, 595–607 (2014).
Roman, C. W., Derkach, V. A. & Palmiter, R. D. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat. Commun. 7, 11905 (2016).
D’Agostino, G. et al. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. Elife 5, e12225 (2016).
D’Agostino, G. et al. Nucleus of the solitary tract serotonin 5-HT2C receptors modulate food intake. Cell Metab. 28, 619–630.e5 (2018).
Aklan, I. et al. NTS catecholamine neurons mediate hypoglycemic hunger via medial hypothalamic feeding pathways. Cell Metab. 31, 313–326.e5 (2020).
Panebianco, M., Rigby, A., Weston, J. & Marson, A. G. Vagus nerve stimulation for partial seizures. Cochrane Database Syst. Rev. 2015, CD002896 (2015).
Carreno, F. R. & Frazer, A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics 14, 716–727 (2017).
Yao, G. et al. Effective weight control via an implanted self-powered vagus nerve stimulation device. Nat. Commun. 9, 5349 (2018).
Pelot, N. A. & Grill, W. M. Effects of vagal neuromodulation on feeding behavior. Brain Res 1693, 180–187 (2018).
Gil, K., Bugajski, A. & Thor, P. Electrical vagus nerve stimulation decreases food consumption and weight gain in rats fed a high-fat diet. J. Physiol. Pharmacol. 62, 637–646 (2011).
Buijs, R. M., Chun, S. J., Niijima, A., Romijn, H. J. & Nagai, K. Parasympathetic and sympathetic control of the pancreas: A role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J. Comp. Neurol. 431, 405–423 (2001).
Browning, K. N. & Travagli, R. A. Plasticity of vagal brainstem circuits in the control of gastric function. Neurogastroenterol. Motil. 22, 1154–1163 (2010).
Blake, C. B. & Smith, B. N. cAMP-dependent insulin modulation of synaptic inhibition in neurons of the dorsal motor nucleus of the vagus is altered in diabetic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R711–R720 (2014).
Derbenev, A. V. & Smith, B. N. Dexamethasone rapidly increases GABA release in the dorsal motor nucleus of the vagus via retrograde messenger-mediated enhancement of TRPV1 activity. PLoS One 8, e70505 (2013).
Boychuk, C. R. et al. A hindbrain inhibitory microcircuit mediates vagally-coordinated glucose regulation. Sci. Rep. 9, 2722 (2019).
Browning, K. N. & Travagli, R. A. Neuropeptide Y and peptide YY inhibit excitatory synaptic transmission in the rat dorsal motor nucleus of the vagus. J. Physiol. 549, 775–785 (2003).
Ju, S. H., Cho, G. B. & Sohn, J. W. Understanding melanocortin-4 receptor control of neuronal circuits: Toward novel therapeutics for obesity syndrome. Pharmacol. Res. 129, 10–19 (2018).
Caverson, M. M., Ciriello, J. & Calaresu, F. R. Paraventricular nucleus of the hypothalamus: an electrophysiological investigation of neurons projecting directly to intermediolateral nucleus in the cat. Brain Res. 305, 380–383 (1984).
Schiöth, H. B., Mutulis, F., Muceniece, R., Prusis, P. & Wikberg, J. E. S. Discovery of novel melanacortin4 receptor selective MSH analogues. Br. J. Pharm. 124, 75–82 (1998).
Gregor Sutcliffe, J. & De Lecea, L. The hypocretins: excitatory neuromodulatory peptides for multiple homeostatic systems, including sleep and feeding. J. Neurosci. Res. 62, 161–168 (2000).
Grabauskas, G. & Moises, H. C. Gastrointestinal-projecting neurones in the dorsal motor nucleus of the vagus exhibit direct and viscreotopically organized sensitivity to orexin. J. Physiol. 549, 37–56 (2003).
Krowicki, Z. K. et al. Orexins in rat dorsal motor nucleus of the vagus potently stimulate gastric motor function. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G465–G472 (2002).
Nakamura, K. et al. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J. Neurosci. 24, 5370–5380 (2004).
Kumagai, H. et al. Importance of rostral ventrolateral medulla neurons in determining efferent sympathetic nerve activity and blood pressure. Hypertens. Res. 35, 132–141 (2012).
Yue, C.-J., Feng, L. & Huang, Q. melanocortinergic-sympathetic signaling: a transneuronal labeling study using pseudorabies virus. Int. J. Clin. Exp. Pathol. 7, 7962–7966 (2014).
Labbé, S. M. et al. Hypothalamic control of brown adipose tissue thermogenesis. Front. Syst. Neurosci. 9, 150 (2015).
Yoshida, K., Li, X., Cano, G., Lazarus, M. & Saper, C. B. Parallel preoptic pathways for thermoregulation. J. Neurosci. 29, 11954–11964 (2009).
Jeong, J. H. et al. Cholinergic neurons in the dorsomedial hypothalamus regulate mouse brown adipose tissue metabolism. Mol. Metab. 4, 483–492 (2015).
Waterhouse, B. D., Devilbiss, D., Seiple, S. & Markowitz, R. Sensorimotor-related discharge of simultaneously recorded, single neurons in the dorsal raphe nucleus of the awake, unrestrained rat. Brain Res. 1000, 183–191 (2004).
Dib, B., Rompré, P. P., Amir, S. & Shizgal, P. Thermogenesis in brown adipose tissue is activated by electrical stimulation of the rat dorsal raphe nucleus. Brain Res. 650, 149–152 (1994).
Gross, P. M. & Weindl, A. Peering through the windows of the brain. J. Cereb. Blood Flow. Metab. 7, 663–672 (1987).
Gross, P. M. Circumventricular organ capillaries. Prog. Brain Res. 91, 219–233 (1992).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Tartaglia, L. A. et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 83, 1263–1271 (1995).
Friedman, J. M. & Halaas, J. L. Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998).
Chua, S. C. et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271, 994–996 (1996).
Spiegelman, B. M. & Flier, J. S. Obesity and the regulation of energy balance. Cell 104, 531–543 (2001).
Gautron, L. & Elmquist, J. K. Sixteen years and counting: an update on leptin in energy balance. J. Clin. Invest. 121, 2087–2093 (2011).
Scott, M. M. et al. Leptin targets in the mouse brain. J. Comp. Neurol. 514, 518–532 (2009).
Patterson, C. M., Leshan, R. L., Jones, J. C. & Myers, M. G. Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res. 1378, 18–28 (2011).
Balthasar, N. et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–991 (2004).
Van De Wall, E. et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149, 1773–1785 (2008).
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011).
Scott, M. M., Williams, K. W., Rossi, J., Lee, C. E. & Elmquist, J. K. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J. Clin. Invest. 121, 2413–2421 (2011).
Zsombok, A. et al. Regulation of leptin receptor-expressing neurons in the brainstem by TRPV1. Physiol. Rep. 2, e12160 (2014).
Williams, K. W., Zsombok, A. & Smith, B. N. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology 148, 1868–1881 (2007).
Cork, S. C. et al. Distribution and characterisation of glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol. Metab. 4, 718–731 (2015).
Wan, S., Coleman, F. H. & Travagli, R. A. Glucagon-like peptide-1 excites pancreas-projecting preganglionic vagal motoneurons. Am. J. Physiol. Gastrointest. Liver Physiol. 292, 1474–1482 (2007).
Blackshaw, L. A. & Grundy, D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J. Auton. Nerv. Syst. 31, 191–201 (1990).
Zheng, Z., Lewis, M. W. & Travagli, R. A. In vitro analysis of the effects of cholecystokinin on rat brain stem motoneurons. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G1066–G1073 (2005).
Wan, S., Coleman, F. H. & Travagli, R. A. Cholecystokinin-8s excites identified rat pancreatic-projecting vagal motoneurons. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G484–G492 (2007).
Mercer, L. D. & Beart, P. M. Histochemistry in rat brain and spinal cord with an antibody directed at the cholecystokinin(A) receptor. Neurosci. Lett. 225, 97–100 (1997).
Glatzle, J., Kreis, M. E., Kawano, K., Raybould, H. E. & Zittel, T. T. Postprandial neuronal activation in the nucleus of the solitary tract is partly mediated by CCK-A receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R222–R229 (2001).
Babic, T. et al. Phenotype of neurons in the nucleus of the solitary tract that express CCK-induced activation of the ERK signaling pathway. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, 845–854 (2009).
Fan, W. et al. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat. Neurosci. 7, 335–336 (2004).
Appleyard, S. M. et al. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J. Neurosci. 25, 3578–3585 (2005).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF-2019R1A2C2005161 to J.-W.S.) funded by the Korean Ministry of Science and ICT.
Author information
Authors and Affiliations
Contributions
U.H. and J.-W.S. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hyun, U., Sohn, JW. Autonomic control of energy balance and glucose homeostasis. Exp Mol Med 54, 370–376 (2022). https://doi.org/10.1038/s12276-021-00705-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-021-00705-9
This article is cited by
-
mTORC1 in energy expenditure: consequences for obesity
Nature Reviews Endocrinology (2024)
-
Association between glycemic variability and short-term mortality in patients with acute kidney injury: a retrospective cohort study of the MIMIC-IV database
Scientific Reports (2024)
-
Reciprocal activity of AgRP and POMC neurons governs coordinated control of feeding and metabolism
Nature Metabolism (2024)
-
Dysregulation of hypoxia-inducible factor 1α in the sympathetic nervous system accelerates diabetic cardiomyopathy
Cardiovascular Diabetology (2023)