Abstract
Invariant natural killer T (iNKT) cells are a major subset of NKT cells that recognize foreign and endogenous lipid antigens presented by CD1d. Although iNKT cells are characteristically autoreactive to self-antigens, the role of iNKT cells in the regulation of cytotoxic T lymphocytes (CTL) has been elucidated using α-galactosylceramide (α-GalCer), a strong synthetic glycolipid that is presented by professional antigen presenting cells (APCs), such as dendritic cells. Despite the well-known effects of α-GalCer and dendritic cells on lipid antigen presentation, the physiological role of endogenous antigens presented by CTLs during crosstalk with iNKT cells has not yet been addressed. In this study, we found that antigen-primed CTLs with transient CD1d upregulation could present lipid self-antigens to activate the iNKT cell production of IFN-γ. CTL-mediated iNKT cell activation in turn enhanced IFN-γ production and the proliferation and cytotoxicity of CTLs. We also found that the direct interaction of iNKT cells and CTLs enhanced the antitumor immune responses of CTLs. This partially explains the functional role of iNKT cells in CTL-mediated antitumor immunity. Our findings suggest that in the absence of exogenous iNKT cell ligands, iNKT cells enhanced the CTL production of IFN-γ and CTL proliferation and cytotoxicity via direct interaction with CD1d expressed on T cells without interacting with APCs.
Similar content being viewed by others
Introduction
Natural killer T (NKT) cells are a subset of TCR αβ cells that recognize self and foreign lipids and glycolipid antigens presented by the MHC class I-like molecule CD1d. Invariant NKT (iNKT) cells represent a major population of cells expressing a unique invariant TCRα chain (Vα14Jα18 in mice and Vα24Jα18 in humans) and a limited number of variable TCRβ chains1. iNKT cells are classified as innate-like lymphocytes that promptly secrete Th1 or Th2 cytokines when antigens bind with TCRs2,3,4. Activated iNKT cells can regulate the adaptive immune response via the recruitment, activation, or modulation of the responses of NK cells, DCs, B cells, and T cells4,5,6,7,8. Many reports have suggested that the activation of iNKT cells enhances the CTL-mediated elimination of cancer cells or various infections9,10,11,12. However, most of these studies were focused on the activation of iNKT cells following stimulation with foreign glycolipids, such as the marine sponge-derived glycolipid α-galactosylceramide (α-GalCer), a strong and prototypical synthetic glycolipid9,10,13,14,15. In these immune responses, activated iNKT cells potentiate dendritic cells (DCs) in a CD40-dependent manner, which in turn enhances the CTL response. Although this mechanism has been well studied, nonphysiological activation using powerful synthetic glycolipid antigens may somehow evoke the differential activation of iNKT cells. Hence, the physiological role of iNKT cells in the immune responses of CTLs remains to be elucidated.
iNKT cells are characteristically autoreactive to self-antigens16 and show characteristic phenotypical activation and memory responses before encountering foreign antigens2,3,16. CD1d is constitutively expressed by many types of cells17. In addition to antigen presenting cells (APCs), T cells also express CD1d18. Tumor-specific CTLs loaded with α-GalCer can recruit and activate iNKT cells to promote CTL-mediated antitumor immune responses19. This suggests that it is possible that mature CD8+ T cells present self-ligands via CD1d to stimulate iNKT cells. Our previous study showed that iNKT cells can promote secondary immune responses of CTLs against tumor cells in the absence of exogenous ligands of iNKT cells20. Nevertheless, it is currently unclear how these homeostatic iNKT cells can strengthen the antigen-specific CTL response. In the present study, we observed that iNKT cells enhanced the effector functions (IFN-γ production, proliferation, and cytotoxicity) of CTLs via the direct recognition of endogenous antigens presented by CD1d on CTLs without the involvement of APCs, such as DCs, or exogenous iNKT cell antigens. Our findings suggest that antigen-stimulated CTLs can activate iNKT cells and cytokines produced by CTL-activated iNKT cells to play a role in the iNKT cell-mediated strengthening of CTL effector function.
Materials and methods
Mice
C57BL/6(B6) wild type (WT) and ovalbumin (OVA)257–264-specific TCR (Vα2 and Vβ5)-expressing transgenic mice (OT-1) were purchased from The Jackson Laboratory. Jα18 knockout (Jα18KO) mice were gifts from Dr. M. Taniguchi (RIKEN, Yokohama, Japan). CD1d knockout mice (CD1dKO) and Vα14 transgenic mice were provided by Dr. Albert Bendelac (University of Chicago, Chicago, IL, USA). CD1dKO mice were crossed with CD1d+/+ (CD1dWT) OT-1 mice to obtain CD1d+/−OT-1 mice. CD1d+/−OT-1 mice were further backcrossed with CD1dKO mice to obtain CD1dKO OT-1 mice. Ly5.1+/− (Ly5.1+Ly5.2+) OT-1 or Ly5.1+/+OT-1 mice were obtained from OT-1 mice that were crossed with congenic Ly5.1+/+ B6 mice. Ly5.1+/− (Ly5.1+Ly5.2+) CD1dKO OT-1 mice were obtained using the same method. All mice exhibited a C57BL/6 background. They were raised in specific pathogen-free conditions at Korea University and used at 7–9 weeks of age. The experimental protocols adopted in this study were approved by the Institutional Animal Care and Use Committee of Korea University.
Cell preparation and enrichment by MACS
Splenic DCs were obtained from splenocytes of CD1dKO mice via magnetic-activated cell sorting (MACS) using anti-CD11c micro beads (Miltenyi Biotec). iNKT cells were purified from Vα14Tg mice using an NK1.1+ iNKT sorting kit (Miltenyi Biotec). CD8+ T cells were positively sorted from the spleens of OT-1 mice via MACS using anti-CD8α micro beads (Miltenyi Biotec) after the depletion of DCs with anti-CD11c magnetic beads (Miltenyi Biotec) according to the manufacturer’s instructions. The purity of the cells sorted by MACS was observed in the following order: DCs > 80%, iNKT cells > 90%, and CD8+ T cells > 98% (Supplementary Fig.1).
CFSE labeling of CD8+ T cells
CD8+ T cells were prepared from the spleens of OT-1 mice by MACS. Purified CD8+ T cells were further resuspended in carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling buffer and labeled with 2.5 μM CFSE via incubation at 37 °C for 10 min followed by extensive washing with RPMI 1640 medium.
Reagents and in vitro stimulation
The cells were cultured in RPMI 1640 medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (HyClone), 2 mM l-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, 10 μg/mL gentamycin sulfate, and 50 μM β-mercaptoethanol. Chicken OVA (OVA257–264 [H-2Kb restricted; amino acid sequence, SIINFEKL) peptide was purchased from Genscript, and the OVA257–264/H-2Kb monomer (hereafter KbOVA) was obtained endogenously. An total of 5 μg/well (50 μl) of KbOVA was incubated at 4 °C overnight in 96-well-flat bottom plates (BD Biosciences).
CD1dKO DCs (1 × 104) were incubated with 10 μg/mL of OVA257–264 peptide for 18 h in a 96-well U bottom plate and washed with RPMI-1640 prior to the stimulation of the OT-1 CD8+ T cells. Purified OT-1 CD8+ T cells (1 × 105) were stimulated with pretreated CD1dKO DCs in the presence or absence of 5 × 104 iNKT cells. Under DC-independent conditions, the purified OT-1 CD8+ T cells were stimulated with plates coated with KbOVA in the presence or absence of 5 × 104 iNKT cells.
In vitro cytotoxic assay
OT-1 CD8+ T cells were stimulated in plates coated with KbOVA for 18 h and cocultured with H3 thymidine-labeled E.G7 cells for an additional 8 h. OVA-expressing E.G7 cells (derivative of EL4) were provided by Dr. M. Mescher (University of Minnesota, Minneapolis, MN, USA). The specific lysis (%) was calculated using the following formula: specific lysis (%) = [(spontaneous CPM − experimental CPM)/spontaneous CPM] × 100.
Flow cytometric (FACS) analysis
Cells were preincubated with anti-FcγR II/III monoclonal antibody (mAb, 2.4G2) and labeled for 30 min with the following mAbs (all mAbs are obtained from DB Biosciences if not specified otherwise): anti-CD8α-PerCP-Cyanine5.5 (clone 53-6.7), anti-CD1d-FITC (clone 1B1), anti-CD11c-FITC (clone HL-3), anti-TCR Vβ5.1-FITC or -APC (clone MR9–4), anti-CD69-FITC (clone H1.2F3), anti-Ly5.1-FITC (clone A20), anti-TCR Vα2-PE (clone B20.1), anti-CD3-PE (clone 17A2), anti-TCRβ-PE or PE-Cy7 (clone H57–597), anti-CD11b-PE (clone M1/70), anti-Ly5.2-PE (clone LG.7F9), CD44-PE (IM7), CD62L-APC(MEL-14), anti-IFNγ-APC (clone XMG1.2), and anti-IL-4-APC (clone 11B11). CD1d dimers were purchased from BD Biosciences, and α-galactosylceramide (α-GalCer) was purchased from Alexis. CD1d/α-GalCer dimers were prepared according to the manufacturer’s protocol.
Stained cells were analyzed on FACSCalibur and FACSVerse flow cytometers (BD Biosciences) using FlowJo software (FlowJo LLC). Figures with panel sets represent analyses performed in the same experiment to facilitate the direct comparison of the fluorescence intensity.
Analysis of intracellular cytokine production
To determine the intracellular cytokine levels, cells were treated with Golgi Stop (BD Pharmingen) for the final 6 h of incubation. The cells were then initially stained for surface molecules with appropriate mAbs, fixed, and permeabilized using a Cytofix/Cytoperm kit (BD Pharmingen). They were finally stained with APC-conjugated anti-IL-4 or anti-IFN-γ mAbs for 45 min on ice. The percentage of cells expressing intracellular IL-4 or IFN-γ was determined via flow cytometry.
OT-1 cell immune responses in vivo and tumor challenge models
Ly5.1+/+ CD1dWT OT-1 CD8+ T cells (1 × 106) and Ly5.1+Ly5.2+ CD1dKO OT-1 CD8+ T cells (1 × 106) were injected into WT (Ly5.2+/+) mice. One day after T-cell injection, the mice were immunized with OVA257–264 peptides/CFA or were not immunized. Two days after immunization, the mice were sacrificed and analyzed.
Sorted CD1dWT OT-1 or CD1dKO OT-1 CD8+ T cells (1 × 105/mouse) were transferred into each recipient mouse via intravenous injection. One day after T cell transfer, the mice were challenged subcutaneously with 1 × 105 E.G7 live tumor cells. Tumor growth was monitored every 3–4 days using digital calipers. The tumor mass was calculated using the following equation: tumor volume (mm3) = (length (cm) × width (cm)2) × 0.5.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0 with either an unpaired t-test or one- or two-way ANOVA with Bonferroni’s multiple comparison test. The bars in all graphs represent the mean ± SEM. The significance is indicated with an asterisk: *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001.
Results
iNKT cells promote CD8+ T-cell activation independent of DCs and exogenous NKT cell agonizts
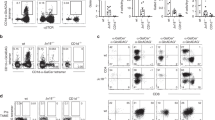
First, we examined whether iNKT cells directly promoted CD8+ T-cell activation or if CD8+ T cells were indirectly activated via interaction with DCs in the absence of exogenous iNKT cell ligands. OT-1 CD8+ T cells (purity > 98%) were stimulated with OVA257–264 peptide-pulsed DCs (purity > 80%) in the presence or absence of iNKT cells (purity > 90%, all purified cells shown in Supplementary Fig. 1). To exclude the possibility of iNKT cell activation by DCs, CD1d knockout DCs (CD1dKO DCs) were used. After 2 days of stimulation, the OT-1 CD8+ T cells secreted higher levels of IFN-γ in the presence of NKT cells than the NKT cell-free culture group (Fig. 1a). Next, we examined whether iNKT cells also affected CD8+ T-cell proliferation using a CFSE dilution assay. The results showed that the proliferation of CTLs was increased when they were cocultured with iNKT cells (Fig. 1b). To exclude the effects of any costimulatory receptors or cytokines produced by CD1dKO DCs on iNKT cells, we stimulated OT-1 CD8+ T cells with artificial APCs [from plates coated with OVA257–264 peptide/H-2Kb monomer (KbOVA)] with or without iNKT cells. The presence of iNKT cells also increased IFN-γ production by OT-1 cells even in the APC-free system (Fig. 1c). These results indicated that iNKT cells directly promoted CTL activation independently of DCs and exogenous iNKT cell ligands.
a Sorted OT-1 CD8+ T cells were activated by CD1d knockout DCs (CD1dKO DCs) pretreated with OVA257–264 peptides in the presence or absence of iNKT cells. After two days of stimulation, the production of IFN-γ by CTLs was examined via intracellular FACS analysis. Detection of IFN-γ-producing CD8+ T cells by staining with anti-TCRVβ5.1, anti-CD8α, and anti-IFN-γ (left panel), b mean fluorescence intensity (MFI) of IFN-γ in OT-1 CD8+ T cells (right panel). c CFSE-labeled OT-1 CD8+ T cells were stimulated as described in (a). Representative histogram displaying CFSE dilution assay of OT-1 cells. d OT-1 CD8+ T cells were stimulated with plates coated with KbOVA in the presence or absence of iNKT cells. After 2 days of stimulation, the amount of IFN-γ produced by OT-1 cells was determined. All data shown are representative of four independent experiments with similar results. Data are presented as the mean ± SEM; **p < 0.01 and ****p < 0.0001. One-way ANOVA with Bonferroni’s multiple comparison test was used for data analysis
Direct interaction of CTLs (CD1d) and NKT cells (TCR) promotes antigen-specific CD8+ T-cell activation
Since CD1d molecules are also expressed on T cells and upregulated upon PHA activation18, we analyzed the kinetic expression of CD1d on CD8+ T cells after TCR priming. The surface levels of CD1d peaked at 18 h after stimulation and declined thereafter (Fig. 2a). To test whether the effect of iNKT cells was mediated via the recognition of CD1d on OT-1 CD8+ T cells, OT-1 CD8+ T cells were pretreated with anti-CD1d mAbs (20H2, 25 μg/mL) before stimulation with KbOVA in the presence of iNKT cells. Pretreatment with anti-CD1d mAbs but not with anti-I-Ek mAb (nonspecific control) abrogated the effect of iNKT cells on the activation of CD8+ T cells (Fig. 2b). This result implied that, without APCs, CTLs were aided directly by iNKT cells via the interaction between CD1d on CTLs and TCRs on iNKT cells.
a OT-1 CD8+ T cells were stimulated with OVA257–264-loaded DCs, and the levels of CD1d were determined by FACS analysis at the indicated time points. b OT-1 CD8+ T cells were stimulated with plates coated with KbOVA in the presence or absence of iNKT cells supplemented with 25 µg/mL of anti-CD1d (20H2) or anti-I-Ek mAbs. After 2 days of stimulation, the levels of IFN-γ secreted by OT-1 cells were determined. c CD1dWT OT-1 CD8+ T cells or CD1dKO OT-1 CD8+ T cells were stimulated with KbOVA in the presence or absence of iNKT cells. After 2 days of stimulation, the levels of IFN-γ produced by OT-1 cells were determined by flow cytometry after intracellular staining. d OT-1 CD8+ T cells were stimulated with KbOVA in the presence or absence of iNKT cells for 18 h and cocultured with H3 thymidine-labeled E.G7 cells for an additional 8 h. The specific lysis (%) was calculated using the following formula: specific lysis (%) = [(spontaneous CPM − experimental CPM)/spontaneous CPM] × 100. All data are representative of at least three independent experiments. Data are presented as the mean ± SEM; **p < 0.01 and ****p < 0.0001. One-way ANOVA with Bonferroni’s multiple comparison test was used for data analysis
To rule out the possibility that DCs that had contaminated the purified iNKT cells stimulated iNKT cells and enhanced OT-1 activation, we compared the effects of iNKT cells on CD1d-expressing wild type OT-1 (CD1dWT OT-1) and CD1d knockout OT-1 (CD1dKO OT-1) CTLs. In the absence of iNKT cells, CD1dKO OT-1 CTLs showed comparable reactivity to their cognate antigen OVA257–264 compared with that of CD1dWT OT-1 CTLs (Fig. 2c). However, when iNKT cells were added to the cultures, the CD1dWT OT-1 CTLs produced significantly higher levels of IFN-γ during KbOVA stimulation compared with the CD1dKO OT-1 CTLs (Fig. 2c). These results indicate that iNKT cells promote IFN-γ production by CTLs via the direct interaction between CTL and iNKT cells, even in the absence of DCs. Next, we investigated whether this direct interaction affected the cytotoxicity of CTLs. The results showed that CD1dWT OT-1 cells exhibited higher cytotoxicity following their interaction with iNKT cells. However, the cytotoxicity of CD1dKO OT-1 cells was not affected by iNKT cells (Fig. 2d).
To determine whether this direct interaction mediated CD8+ T cell immunity in vivo, the priming of CD8+ T cells was analyzed using congenic mice. We co-injected naïve CD1dKO OT-1 cells (Ly5.1+Ly5.2+) and CD1dWT OT-1 cells (Ly5.1+/+) into WT (Ly5.2+/+) mice and immunized the mice with OVA257–264 peptides. After 2 days of antigen-specific activation, the number of CD1dWT OT-1 cells was slightly higher than that of CD1dKO OT-1 cells in peripheral blood and spleen (Fig. 3a, b). There was no significant difference between the two populations in the inguinal lymph nodes (Fig. 3a, b), where the NKT cell population size was very small21. The difference in the cell proliferation of CD1dWT OT-1 cells and CD1dKO OT-1 cells was not as large as that observed under in vitro stimulation (Fig. 2b). This might be due to the lower abundance of iNKT cells in the in vivo environment compared to that under in vitro stimulation.
Equal numbers of purified CD8+ T cells from Ly5.1+/+ CD1dWT OT-1 splenocytes and Ly5.1+ Ly5.2+ CD1dKO OT-1 splenocytes were i.v. cotransferred to Ly5.2+/+WT C57BL6/c mice (n = 6). After 1 day, 4 of 6 mice were stimulated with OVA257–264 peptides. After 2 days of stimulation, the transferred cells in peripheral blood (PBL), spleen (SP), and inguinal lymph nodes (ILN) were analyzed. a Representative FACS data show the frequencies of the donor cells. b The fold changes in donor cells were calculated by dividing the frequencies of donor cells in immunized mice by those in unimmunized mice. Each dot represents a recipient. Data are representative of two independent experiments. Data are presented as the mean ± SEM; *p < 0.05 and ***p < 0.0001. Unpaired t test was used for statistical analysis
Activated CTLs can activate iNKT cells, which in turn promote CTL activation
iNKT cells are activated by endogenous lipid antigens presented by DCs in the presence of pro-inflammatory cytokines22,23, which prompted us to investigate the presentation of endogenous antigens by stimulated CTLs via CD1d to activate iNKT cells. First, we investigated whether OT-1 CD8+ T cells could activate iNKT cells via endogenous antigens presented on CD1d. iNKT cells were cocultured with CD1dWT OT-1 or CD1dKO OT-1 cells. The direct interaction between CD1d (CTLs) and TCR (iNKT) enhanced the iNKT cell production of IFN-γ but not of IL-4, another representative cytokine produced by iNKT cells (Fig. 4a). Next, we determined whether both naïve and primed CTLs activated iNKT cells. IFN-γ production and CD69 expression by iNKT cells were examined after coculture with naïve or activated OT-1 CD8+ T cells. We found that primed but not naïve CD8+ T cells activated iNKT cells (Fig. 4b, c). Taken together, these results suggest that primed CTLs present endogenous antigens via CD1d to activate iNKT cells to a certain degree.
a Purified CD1dWT OT-1 or CD1dKO OT-1 CD8+ T cells were stimulated with KbOVA in the presence of iNKT cells. The percentages of IFN-γ- or IL-4-producing iNKT cells were examined using intracellular FACS analysis. b Naïve OT-1 CD8+ T cells were stimulated with KbOVA for 18 h. iNKT cells were cocultured with preactivated or naïve OT-1 cells. The levels of IFN-γ in iNKT cells were evaluated after 2 h of culture using intracellular FACS analysis (b). c Purified OT-1 CD8+ T cells and iNKT cells were cocultured with CD1dKO DCs in the presence or absence of OVA257–264. After 18 h of activation, the surface expression of CD69 on iNKT cells was measured by FACS. iNKT cells were gated on the α-GalCer-CD1d dimer+ TCR-β+ cell population. All data are representative of at least three independent experiments. Data are presented as the mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001. Unpaired t tests (a, c). One-way ANOVA with Bonferroni’s multiple comparison test was used for statistical analysis (b)
Next, we explored whether IFN-γ produced by iNKT was responsible for the iNKT cell-mediated enhancement of CTL effector function. The CTL response was observed by blocking cytokine expression. The abrogation of IFN-γ significantly reduced the iNKT cell-mediated increase in the CTL production of IFN-γ. However, blocking IL-4 or IL-12 did not affect the intracellular level of IFN-γ in CTLs (Fig. 5a). It appeared that IFN-γ played a role in the iNKT cell-mediated promotion of CTL function. However, CTLs themselves also synthesize IFN-γ. Blocking IFN-γ may also affect CTLs. Therefore, we activated populations of CD1dKO (Ly5.2+/+) OT-1 and CD1dWT (Ly5.1+Ly5.2+) OT-1 cells in the presence or absence of iNKT cells to determine whether cytokines released by CD1dKO OT-1 cells activated iNKT cells to affect the CD1dKO OT-1 cell production of IFN-γ. In the absence of iNKT cells, both CD1dWT and CD1dKO OT-1 cells showed similar IFN-γ production levels. However, the production of IFN-γ by CD1dWT OT-1(Ly5.1+) cells was higher than that by CD1dKO OT-1(Ly5.1−) cells in the presence of iNKT cells. In addition, the presence of iNKT cells increased the production of IFN-γ by CD1dKO OT-1 but not as strongly as that by CD1dWT OT-1 (Fig. 5b) because iNKT cells produced cytokines that influenced CD1dKO OT-1 cells nonspecifically through a bystander effect, since both CD1dWT OT-1 and CD1dKO OT-1 cells were mixed in this experimental setting. These results suggested that the interaction between activated CTLs and iNKT cells depended specifically on the CD1d-TCR interaction. CTL-activated iNKT cells played a role in CTL function mediated by local soluble factors (mainly IFN-γ) via intimate physical contact with CTLs.
a OT-1 cells were cocultured with OVA257–264-unloaded or -loaded CD1dKO DCs in the presence or absence of iNKT cells. After 12 h of stimulation, the indicated blocking monoclonal antibodies were added, and the cells were incubated for another 24 h. The levels of IFN-γ in OT-1 cells were examined using intracellular FACS analysis. b Equal numbers of Ly5.1+Ly5.2+CD1dWT OT-1 and Ly5.2+Ly5.2+CD1dKO OT-1 CD8+ T cells were mixed and stimulated with KbOVA in the presence or absence of iNKT cells. After 2 days of stimulation, the levels of IFN-γ in OT-1 cells were determined using intracellular FACS analysis. b MFI of the intracellular IFN-γ levels of CD1dWT OT-1 or CD1dKO OT-1 cells gated on Ly5.1+ (CD1dWT OT-1) or Ly5.1− (CD1dKO OT-1) Vα2+CD8+ T cells. All data are representative of at least three independent experiments. Data are presented as the mean ± SEM. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. One-way ANOVA with Bonferroni’s multiple comparison test was used for statistical analysis
Direct interaction promotes CTL-mediated antitumor effects
Our previous study has shown that homeostatic iNKT cells can enhance CTL-mediated antitumor activity20. Here, we examined whether the direct interaction between CTLs and iNKT cells strengthened the CTL-mediated antitumor response. Sorted naïve CD1dKO OT-1 cells or CD1dWT OT-1 cells were transferred to wild type C57BL/6 mice, in which endogenous iNKT cells exist, followed by the injection of E.G7 tumor cells. CD1dWT OT-1 cells efficiently controlled the growth of tumor cells. However, CD1dKO OT-1 cells did not suppress tumor growth as efficiently as CD1dWT OT-1 cells (Fig. 6a). To further establish that iNKT cells increased CD1dWT OT-1 cell-mediated suppression of tumor growth, we performed the same experiments in Jα18KO recipient mice, which lack iNKT cells. The results showed that the absence of iNKT cells eliminated the predominant tumor regression mediated by CD1dWT OT-1 cells (Fig. 6b). Therefore, iNKT cells enhanced the antitumor effects of CTLs partially via the direct interaction of CTLs and iNKT cells.
CD1dWT OT-1 CD8+ T cells or CD1dKO OT-1 CD8+ T cells were intravenously transferred to wild type (a) and Jα18KO NKT cell-deficient (b) mice (n = 5). Tumor growth was monitored every 2–4 days. The data shown are representative of two independent experiments with similar results. Data are presented as the mean ± SEM. **p < 0.01, ***p < 0.001; two-way ANOVA with Bonferroni’s multiple comparison test
Discussion
NKT cells exhibiting both innate and adaptive immune functions play an antitumor role. They can also suppress infections10,11,12. The iNKT cell-mediated modulation of CTL activity is generally considered to occur via indirect regulation by DCs9. iNKT cells are activated by the CD1d-glycolipid complex presented by DCs and in turn induce the maturation of DCs to cross-prime CD8+ T cells. However, CD1d expression is not restricted to APCs. The expression of CD1d on CD8+ T cells is elevated after activation, indicating that CD1d expression on CD8+ T cells may play a role in the crosstalk between iNKT and CD8+ T cells. In the current study, we found that iNKT cells also promoted CD8+ T cell activation in a DC-independent manner, even in the absence of exogenous antigens (Fig. 1). Using CD1dKO OT-1 cells, we found that iNKT cells directly interacted with CD1d on CTLs, which boosted IFN-γ synthesis by CTLs, cell proliferation, and cytotoxicity (Fig. 2). In addition, we found that the upregulation of CD1d expression on activated CD8+ T cells presenting endogenous antigens modestly activated iNKT cells to secrete IFN-γ, which might play a role in the enhancement of CTL function. Furthermore, we found that this direct interaction enhanced CTL proliferation and CTL-mediated antitumor immunity in in vivo CTL activation and established tumor models (Fig. 3 and Fig. 5). Therefore, the direct effect of iNKT cells on CD8+ T-cell immunity partially explained the physiological role of iNKT cells.
Several studies have shown that endogenous lipid antigens can activate peripheral iNKT cells22,24,25,26. The endogenous lipid antigens of iNKT cells have a diverse nature and are generally divided into glycosphingolipids and phospholipids16,25,27. Several candidate endogenous lipid antigens have been proposed, including isoglobotrihexosylceramide (iGb3)16, β-glucosylceramide(β-GlcCer)24,28, α-glycosylceramides27, the peroxisome-derived ether-bonded compound lysophosphatidylethanolamine (pLPE), and lysophosphatidic acid (eLPA)29. All of these lipid self-antigens that have been experimentally proposed to be involved in NKT cell activation are presented by APCs (DCs and macrophages). CTLs might also present these lipid candidates to activate iNKT cells. In fact, we found that CD1dWT CTLs could activate iNKT cells, while CD1dKO CTLs failed to activate iNKT cells, indicating that CTLs could activate iNKT cells by presenting endogenous antigens (Fig. 4).
In addition, the induction of CD1d may be important for augmenting NKT cell activation by weak endogenous antigens30. As shown in Fig. 2a, CTLs showed the transient elevation of CD1d expression after TCR engagement. Primed CTLs with a higher level of CD1d increased iNKT cell production of IFN-γ, indicating that the upregulation of CD1d might facilitate the presentation of endogenous antigens to iNKT cells via the additional synthesis of cytokines such as IL-2 and IFN-γ (Fig. 4a). The engagement of CD1d by CTLs enhanced the production of IFN-γ but not the production of IL-4 by iNKT cells (Fig. 4a). This modest effect on activation is consistent with the indirect activation of iNKT cells by DCs23.
iNKT cells play a significant role in the rapid production of cytokines to facilitate adaptive immunity6,7. Therefore, we investigated whether cytokines produced by CTL-activated iNKT cells enhanced CTL function. As shown in Fig. 5a, blocking IFN-γ but not IL-4 or IL-12 abolished the iNKT cell-induced increase in IFN-γ levels in CTLs. However, CTLs themselves also produced IFN-γ. Therefore, we generated a specific cytokine environment by using CD1dWT OT-1 cell-activated iNKT cells and CD1dKO OT-1 cells via coculturing these two types of OT-1 cells in the presence of iNKT cells. As shown in Fig. 5b, iNKT cells not only enhanced CD1dWT OT-1 cell production of IFN-γ but also slightly enhanced production by CD1dKO OT-1 cells, suggesting that the iNKT cell-mediated enhancement of CTL effector function was specifically induced by CD1d-dependent cell–cell contact. The slight increase in IFN-γ production by CD1dKO OT-1 cells in the coculture system was attributed to the bystander effect of cytokines produced by iNKT cells. Figure 5a, b indicates that among the cytokines produced by iNKT cells, IFN-γ most likely enhanced CTL effector function, including the motility and cytotoxicity of CTLs31.
iNKT cells can interact with CD1d molecules17. This not only results in iNKT cell activation but also induces the phosphorylation of CD1d, intracellular signaling, and the release of cytokines from CD1d-bearing cells32. Hence, it is possible that the intracellular signaling of CD1d-expressing T cells is enhanced after interaction with iNKT cells.
Taken together, our findings suggest that CD8+ T cells are activated via the recognition of specific antigens on APCs or target cells, leading to the upregulation of CD1d on activated CTLs. iNKT cells can directly interact with CD1d expressed on CTLs and boost the function of CTLs via the upregulation of IFN-γ production, cell proliferation, and cytotoxicity, suggesting that CD8+ T cells can reach maximal reactivity through a dual mechanism first involving the engagement of APCs followed by interaction with iNKT cells (Fig. 7).
CD8+ T cells upregulate the expression of CD1d on their surfaces upon the recognition of cognate antigens (1). iNKT cells interact with CD1d on antigen-recognized CD8+ T cells to become activated (2) and to further stimulate CD8+ T cells in turn (3). CD8+ T cells reach the optimal level of activation and downregulate CD1d expression on the cell surface (4)
References
Rossjohn, J., Pellicci, D. G., Patel, O., Gapin, L. & Godfrey, D. I. Recognition of CD1d-restricted antigens by natural killer T cells. Nat. Rev. Immunol. 12, 845–857 (2012).
Kronenberg, M. & Gapin, L. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2, 557–568 (2002).
Godfrey, D. I., MacDonald, H. R., Kronenberg, M., Smyth, M. J. & Van Kaer, L. NKT cells: what’s in a name? Nat. Rev. Immunol. 4, 231–237 (2004).
SJ, HermansI. F. et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol. 171, 5140–5147 (2003).
Carnaud, C. et al. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163, 4647–4650 (1996).
Kitamura, H. et al. alpha-galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell Immunol. 199, 37–42 (2000).
Fujii, S. S. K., Smith, C., Bonifaz, L. & Steinman, R. M. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 198, 267–279 (2003).
Doherty, D. G., Melo, A. M., Moreno-Olivera, A. & Solomos, A. C. Activation and regulation of B cell responses by invariant natural killer T cells. Front. Immunol. 9, 1360 (2018).
Gottschalk, C., Mettke, E. & Kurts, C. The role of invariant natural killer T cells in dendritic cell licensing, cross-Priming, and memory CD8(+) T cell generation. Front. Immunol. 6, 379 (2015).
King, L. A., Lameris, R., de Gruijl, T. D. & van der Vliet, H. J. CD1d-invariant natural killer T cell-based cancer immunotherapy: α-Galactosylceramide and beyond. Front. Immunol. 9, 1519, https://doi.org/10.3389/fimmu.2018.01519 (2018).
Araki, M., Miyake, S. & Yamamura, T. Synthetic glycolipid ligands for human iNKT cells as potential therapeutic agents for immunotherapy. Curr. Med. Chem. 15, 2337–2345 (2008).
Behar, M. S. & Skold, M. Role of CD1d-restricted NKT cells in microbial immunity. Infect. Immun. 71, 5447–5455 (2003).
Semmling, V. et al. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat. Immunol. 11, 313–320 (2010).
Ghinnagow, R. et al. Co-delivery of the NKT agonist α-galactosylceramide and tumor antigens to cross-priming dendritic cells breaks tolerance to self-antigens and promotes antitumor responses. Oncoimmunology 6, e1339855 (2017).
Yamashita, K. et al. Application of iNKT cell-targeted active immunotherapy in cancer treatment. Anticancer Res. 38, 4233–4239 (2018).
Zhou, D. et al. Lysosomal glycosphingolipid recognition by NKT cells. Science 306, 1786–1789 (2004).
Brigl, M. & Brenner, M. B. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22, 817–890 (2004).
Salamone, M. C., Rabinovich, G. A., Mendiguren, A. K., Salamone, G. V. & Fainboim, L. Activation-induced expression of CD1d antigen on mature T cells. J. Leukoc. Biol. 69, 207–214 (2001).
Choi, D. H. et al. Dendritic cell internalization of α-galactosylceramide from CD8 T cells induces potent antitumor CD8 T-cell responses. Cancer Res. 71, 7442–7451 (2011).
Hong, C. et al. Regulation of secondary antigen-specific CD8(+) T-cell responses by natural killer T cells. Cancer Res. 69, 4301–4308 (2009).
Slauenwhite, D. & Johnston, B. Regulation of NKT cell localization in homeostasis and infection. Front. Immunol. 6, 255 (2015).
Mattner, J. et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005).
Brigl, M., Bry, L., Kent, S. C., Gumperz, J. E. & Brenner, M. B. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 4, 1230–1237 (2003).
Brennan, P. J. et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat. Immunol. 12, 1202–1211 (2011).
Pei, B. et al. Diverse endogenous antigens for mouse NKT cells: self-antigens that are not glycosphingolipids. J. Immunol. 186, 1348–1360 (2010).
Brennan, P. J. et al. Activation of iNKT cells by a distinct constituent of the endogenous glucosylceramide fraction. Proc. Natl Acad. Sci. USA 111, 13433–13438 (2014).
Darmoise, A. et al. Lysosomal alpha-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity 33, 216–228 (2010).
Paget, C. et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity 27, 597–609 (2007).
Federica, F. et al. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat. Immunol. 13, 474–480 (2012).
Sköld, M., Xiong, X., Illarionov, P. A., Besra, G. S. & Behar, S. M. Interplay of cytokines and microbial signals in regulation of CD1d expression and NKT cell activation. J. Immunol. 175, 3584–3593 (2005).
Bhat, P., Leggatt, G., Waterhouse, N. & Frazer, I. H. Interferon-γ derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis. 8, e2836 (2017).
Kawana, K. et al. Expression of CD1d and ligand-induced cytokine production are tissue specific in mucosal epithelia of the human lower reproductive tract. Infect. Immun. 76, 3011–3018 (2008).
Acknowledgements
The authors thank the staff of Gyerim Experimental Animal Resource Center for animal care and technical assistance.
Funding
This work was supported by a grant (NRF-2018R1A2A2A05023297) from the Basic Science Research Program of the National Research Foundation of Korea.
Author information
Authors and Affiliations
Contributions
S.O., Y.Q. and S.-H.P. designed the experiments; S.O, Y.Q. and S.L. performed the experiments; S.O., Y.Q, J.H.S., M.S.Y. and S.-H.P analyzed the data; Y.Q. and S.O. wrote the paper; S.-H.P. supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qin, Y., Oh, S., Lim, S. et al. Invariant NKT cells facilitate cytotoxic T-cell activation via direct recognition of CD1d on T cells. Exp Mol Med 51, 1–9 (2019). https://doi.org/10.1038/s12276-019-0329-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-019-0329-9
This article is cited by
-
Motility and tumor infiltration are key aspects of invariant natural killer T cell anti-tumor function
Nature Communications (2024)
-
CAR-NKT cell therapy: a new promising paradigm of cancer immunotherapy
Cancer Cell International (2023)
-
Monocytic myeloid-derived suppressive cells mitigate over-adipogenesis of bone marrow microenvironment in aplastic anemia by inhibiting CD8+ T cells
Cell Death & Disease (2022)
-
NK and cells with NK-like activities in cancer immunotherapy-clinical perspectives
Medical Oncology (2022)
-
Role of NKT cells in cancer immunotherapy—from bench to bed
Medical Oncology (2022)