Abstract
Acetate has been indicated to be elevated and to regulate inflammation in inflammatory and metabolic diseases. The inflammasome serves as a key component of immune homeostasis, and its dysregulation can lead to various inflammatory disorders. However, little is known about the effects of acetate on inflammasome activation and the underlying mechanism. Here, we demonstrate that acetate attenuates inflammasome activation via GPR43 in a Ca2+-dependent manner. Through binding to GPR43, acetate activates the Gq/11 subunit and subsequent phospholipase C-IP3 signaling to decrease Ca2+ mobilization. In addition, acetate activates soluble adenylyl cyclase (sAC), promotes NLRP3 inflammasome ubiquitination by PKA, and ultimately induces NLRP3 degradation through autophagy. In vivo, acetate protects mice from NLRP3 inflammasome-dependent peritonitis and LPS-induced endotoxemia. Collectively, our research demonstrates that acetate regulates the NLRP3 inflammasome via GPR43 and Ca2+-dependent mechanisms, which reveals the mechanism of metabolite-mediated NLRP3 inflammasome attenuation and highlights acetate as a possible therapeutic strategy for NLRP3 inflammasome-related diseases.
Similar content being viewed by others
Introduction
The inflammasome is a series of multiprotein complexes that serve as a platform to promote interleukin (IL)-1β secretion and pyroptosis1. The NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome comprises the NOD-like receptor, the adaptor protein ASC and caspase-1 and is considered to play a crucial role in the immune response to pathogens2. However, dysregulation of NLRP3 inflammasome activation leads to excessive inflammation, which is linked to various inflammatory disorders, including type 2 diabetes, atherosclerosis, gout, and Alzheimer’s disease3,4,5. Therefore, the regulation of NLRP3 inflammasome activation has emerged as a therapeutic target for inflammasome-related illnesses.
Recently, several metabolites, such as short-chain fatty acids, dopamine and bile acid, have been reported to be involved in NLRP3 inflammasome regulation5,6,7. For example, by binding to transmembrane G-protein-coupled receptor-5 (TGR5) or dopamine receptor D1 (DRD1), metabolites can induce an increase in cyclic adenosine monophosphate (cAMP) and subsequently activate protein kinase A (PKA) and promote NLRP3 ubiquitination and degradation through autophagy or the proteasome5,6. cAMP generated by transmembrane or soluble adenylyl cyclase (tmAC or sAC, respectively) is considered to function as a brake for inflammasome activation5,6,8. However, it remains unknown whether other metabolites function in a similar manner.
Acetate is a short-chain fatty acid produced by the gut microbiota, and its effects9,10,11,12, when elevated in specific conditions, are extensive and include modulation of tumor growth and regulation of the endocrine and cardiovascular systems. Gao et al.9 regard acetate to be an epigenetic modulator that promotes lipid synthesis in cancer cells. Another study showed that increased concentrations of acetate promoted metabolic syndrome in a parasympathetic signaling-dependent manner10. In most cases, the biological effect of acetate depends on its receptor13. G-protein-coupled receptor 43 (GPR43) is widely expressed on the surface of innate immune cells, including macrophages, and engages in immune activity. For example, in polymorphonuclear cells, GPR43 functions as a regulator that modulates the activity of neutrophils14. GPR43 is a natural receptor for acetate15, but most studies have focused on acetate in the context of the gut microbiota and the regulation of inflammatory homeostasis13,16. Despite the emerging importance of acetate in the regulation of inflammation, the specific function of acetate in inflammasome regulation and the underlying mechanism remain largely unknown.
Our investigation demonstrates that acetate regulates the NLRP3 inflammasome via GPR43-sAC-PKA signaling and attenuates its activation through ubiquitination and autophagy. Furthermore, acetate also shows anti-inflammatory effects on several peritonitis and endotoxemia models in vivo, indicating that acetate might represent an attractive strategy for treating inflammasome-related diseases.
Materials and methods
Animal model and experiments
Six- to 8-week-old male C57BL/6J mice were purchased from the Shanghai Laboratory Animal Center (SLAC) (China). All mice were housed at a temperature of 18–22 °C with a relative humidity of 50–60% and a 12-h light–dark cycle, with free access to water and food. Animal experiments were approved by the Scientific Investigation Board of Second Military Medical University. For the mouse peritonitis model, each mouse was pretreated with 40 µl of 2.5 M acetate (acetate solution, pH 5.2, Sigma-Aldrich, Lot. 3863) diluted in 400 µl of PBS. Thirty minutes later, the mice were injected with alum (2 mg/mouse; Thermo Scientific, China) or monosodium urate (MSU) (2 mg/mouse; Sigma, China) diluted in 50 µl of PBS or with 10 mg/kg lipopolysaccharide (LPS) (Escherichia coli O111:B4, Sigma, China) diluted in 300 µl of PBS; 6 h after the MSU or alum injection, peritoneal lavage was performed with 5 ml of normal saline (NS), and the lavage fluid was centrifuged.
Cell preparation and stimulation
For peritoneal macrophage (PM) generation, each mouse was injected with thioglycollate (BD, USA). Three days after the injection, peritoneal lavage was performed with 5 ml of NS. The cells were resuspended at 2–4 × 106 cells/ml and cultured in RPMI 1640 culture medium supplemented with 10% fetal bovine serum (FBS). For bone marrow-derived macrophage (BMDM) generation, mouse femoral tissue was isolated, and the bone marrow was flushed with 3 ml of NS. After red blood cell lysis, bone marrow cells were resuspended at 2–4 × 106 cells/ml in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and 30 ng/ml GM-CSF (PeproTech, USA). The culture medium was changed every 2 days, and after 5–6 days of culture, the cells were subjected to further experiments. To activate the inflammasome in PMs or BMDMs, the cells were first primed with LPS (100 ng/ml) for 3 h, and ATP (5 mM), nigericin (20 µM), muramyl dipeptide (MDP) (200 ng/ml), flagellin (10 µM) or poly(dA:dT) (1 µg/ml) was added for 30 min. The supernatant was subjected to further analysis. Inhibitors (KH7, H89 and bafilomycin A1 from Cayman Chemical, USA; MG-132 from Selleck, USA; and 3-MA from Merck, Germany) or agonists (GPR43 agonist, Millipore, USA) were added to the culture medium 30 min before acetate pretreatment and after LPS priming.
Cytokine ELISA, lactate dehydrogenase (LDH) assay, and cAMP measurement
IL-1β, IL-6, and tumor necrosis factor (TNF)-α were analyzed using an ELISA kit purchased from Invitrogen (USA), and IL-18 was analyzed using a kit from R&D Systems (USA). LDH release assays were performed using a Cytotoxicity Detection Kit purchased from Roche Life Science (USA). cAMP measurement was performed by using a cAMP Parameter Assay Kit (R&D Systems, USA).
Immunoblotting and immunoprecipitation
Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (Beyotime, China), and protein concentrations were determined using a BCA assay (Thermo, China). The total protein samples (approximately 20 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride (PVDF) membranes (Merck, Germany), and blocked with 5% nonfat dry milk in phosphate-buffered saline with Tween (PBST), pH 7.5. The membranes were immunoblotted with primary antibodies for 4 h or overnight at 4 °C and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. The anti-ASC, anti-NLRP3, anti-p62, anti-LC3B, anti-β-actin, anti-K63, anti-K48, anti-pro-IL-1β, anti-phospho-IKKβ, anti-IKKβ, anti-phospho-p65, and anti-p65 antibodies were purchased from Cell Signaling Technology (MA, USA). Other antibodies used in the present study were as follows: anti-pro-caspase-1 (Adipogen, USA), anti-GPR43 (Absin, China), anti-GPR41 (Thermo Fisher, USA), anti-MARCH7 (Bioss, China), and anti-sAC (FabGennix, USA). The HRP-conjugated secondary antibodies were all purchased from Cell Signaling Technology (USA). Protein bands were detected with an enhanced chemiluminescence kit (Pierce, USA).
For the immunoprecipitation assay, briefly, the cells were collected and lysed using a mortar and pestle in a buffer containing 20 mM PIPES, pH 6.8, 1% Triton X-100, 150 mM NaCl, 150 mM sucrose, 0.2% sodium deoxycholate, 500 μM EDTA and protease inhibitors for 5 min on ice. After centrifugation, the supernatants were diluted to 2 μg/ml in dilution buffer containing 20 mM PIPES, pH 6.8, 1% Triton X-100, 150 mM NaCl, 150 mM sucrose, 2.5 mM MgCl2 and 2.5 mM MnCl2. Primary antibody-conjugated protein A beads were incubated with the lysates for 2 h at 4 °C before washing with dilution buffer. The subsequent immunoblot analysis was conducted using the abovementioned methods. The chemicals were purchased from Sigma.
Flow cytometry
Peritoneal lavage cells from individual mice were prepared in PBS and stained with antibodies at 4 °C for 15 min. After washing three times, the cells were resuspended in washing buffer and analyzed. Fluorescence data for 105 events from each sample were acquired on a FACS LSR II (BD Bioscience, USA) and analyzed using FlowJo software (Tomy Digital Biology Co., Ltd., Japan). The antibodies used in these experiments (neutrophils: anti-mouse-CD11b and anti-mouse-GR-1; macrophages: anti-mouse-F4/80) were purchased from eBioscience or Invitrogen (USA).
Short-interfering RNA (siRNA) interference
Mouse PMs or BMDMs were cultured in half of the final total culture volume in FBS-free RPMI 1640 or DMEM and transfected with 3 ng/ml siRNA (si-GPR43, si-GPR41, si-MARCH7, si-sAC1, si-sAC2, or si-Gq) or control siRNA (GenePharma, China) by INTERFEREin (Invitrogen, USA) for 6 h according to the manufacturer’s instructions. Six hours later, the other half of the complete culture medium was added, and the cells were cultured for a total of 48 h. After 48 h of interference, the cells were subjected to further stimulation or experiments. The sequences of each siRNA were as follows: si-GPR43: GCUGGUACCUACCAAAGAUdTdT; si-GPR41: GCUUCUUUCUUGGCAAUUAdTdT; si-MARCH7: GGACUUAUGUAGAAUUUGUdTdT; si-sAC1: AAUGUAUGGGCUUCAUGGAdTdT; si-sAC2: TCGGAGCATGATTGAAATCGA; and si-Gq: AAAUGACACUUUGUAAGUCAAAGGG.
Cytosolic calcium analysis
Macrophages were primed with 100 ng/ml LPS (E. coli O111:B4, Sigma, China) for 3 h. After two washes, the cells were incubated in HBSS containing 2 µM Fluo 4-AM (Life Technologies, USA) for 30 min in the dark. The cells were then washed, treated with or without acetate (20 mM) for 30 min, treated with 2-APB (50 µM) (Sigma-Aldrich, USA) for 15 min, resuspended in HBSS and added to black 96-well plates (Corning Costar, USA). After 10 min of incubation, the cells were stimulated with nigericin (20 µM) (Sigma-Aldrich, USA). A DMI6000 inverted fluorescence microscope (Leica, Germany) with an X-Cite® 200DC fluorescent light source (Lumen Dynamic, Canada) was used for fluorescence analysis. Fluorescence emission at 520 nm and excitation at 485 nm were used. ΔFluorescence was calculated as Fmax (fluorescence of the calcium-saturated indicator) minus F0 (fluorescence in nonstimulated cells in HBSS).
Statistical analysis
Data were expressed as the mean ± SEM. Student’s t-test was conducted for comparisons between two groups, and one-way ANOVA was performed for comparisons among several groups. A P-value < 0.05 was considered to be statistically significant.
Results
Acetate attenuates NLRP3-mediated inflammasome activation in vitro and in vivo
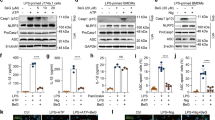
To explore the anti-inflammatory effect of acetate on NLRP3 inflammasome activation, LPS-primed BMDMs were treated with acetate before nigericin stimulation. The concentrations of IL-1β and IL-18 in the supernatant were tested. We found that acetate suppressed the ATP- or nigericin-induced production of IL-1β and IL-18 in a dose-dependent manner (Fig. 1a–d). In addition, the attenuation of cytokine production could also be observed when acetate was added after LPS and nigericin activation (Fig. 1e). The acetate treatment had little effect on LDH production (Supplemental Fig. 1), which suggests that the inhibitory effect was not due to damage to the cells.
a–d ELISA of IL-1β (a, b) and IL-18 (c, d) in BMDMs treated with LPS + nigericin or LPS + ATP at different doses (20, 30, 35, 40, and 60 mM) (n = 3); e ELISA of IL-1β after sodium acetate treatment (n = 3); f ELISA of IL-1β at different time points after acetate treatment in LPS-primed ATP-treated BMDMs (n = 3); n = biological replicates; Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, Student’s t-test compared with the control group
We further performed comprehensive experiments investigating the role of acetate in suppressing inflammation and the inflammasome. We found that acetate suppressed IL-1β and IL-18 production in response to pretreatment, simultaneous treatment or post treatment with LPS and nigericin (Fig. 2a, b). Moreover, acetate decreased TNF-α and IL-6 production by LPS-primed BMDMs (Fig. 2c, d) and partly attenuated NF-κB signaling activation accordingly (Supplemental Fig. 2). In the further analysis of NLRP3 inflammasome activation in response to acetate pretreatment, we found that acetate could decrease the level of cleaved caspase-1 (p20) in the supernatant and cell lysate of BMDMs and NLRP3 in cell lysates (Fig. 2e), indicating the inhibitory effect of acetate on NLRP3 inflammasome activation.
a–d ELISA of IL-1β (a), IL-18 (b), TNF-α (c), and IL-6 (d) production after acetate treatment in BMDMs. Acetate (10, 20, and 30 mM) was administered 30 min before LPS priming, 30 min before nigericin stimulation, simultaneously with nigericin or 30 min after nigericin stimulation. All treatments showed suppressive effects in a dose-dependent manner (n = 3); *P < 0.05, **P < 0.01, and ***P < 0.001, Student’s t-test compared with the LPS + Nig group; #P < 0.05, ##P < 0.01, and ###P < 0.001, one-way ANOVA for comparison. e Western blot of pro-caspase-1, caspase-1-p20, and IL-1β-p17 in supernatant and NLRP3, pro-caspase-1, caspase-1-p20 and β-actin in cell lysate after indicated treatment; f–h ELISA of IL-1β after treatment with poly(dA/dT), MDP and flagellin (n = 3). n = biological replicates. Data are presented as the mean ± SEM
We also determined that acetic acid, rather than hydrogen ions, exerts these inhibitory effects because sodium acetate had an inhibitory effect similar to that of acetate (Fig. 1f). These results indicated that the suppression mediated by acetate was independent of the treatment period, which suggests that acetate exerted its inhibitory effect on the inflammasome during the activation stage.
Different inflammasomes respond to different signals and trigger different responses17. To determine whether acetate impacts other types of inflammasomes, we tested the impact of acetate on the Absent in melanoma 2 (AIM2), NLR family CARD domain-containing protein 4 (NLRC4) and NACHT, LRR and PYD domain-containing protein 3 (NALP3) inflammasomes after activation by poly(dA:dT), flagellin or MDP. Acetate showed no detectable inhibitory effect (NLRC4 and NALP3) or relatively limited attenuation (AIM2) on these inflammasomes (Fig. 2f, g). Taken together, our results show that acetate suppresses IL-1β production mediated by the NLRP3 inflammasome.
We further evaluated the anti-inflammatory effect of acetate in vivo. To adapt MSU- and alum-induced peritoneal inflammation3,18, which have been proven to be NLRP3 inflammasome-dependent models, and LPS-induced peritonitis, we pretreated mice with acetate intraperitoneally to investigate its anti-inflammatory effects. Peritoneal lavage fluid (PLF), peritoneal exudate cells (PECs) and serum were harvested for further assessments. As shown in Fig. 3 and Supplemental Fig. 3, compared with those of the control mice, IL-1β levels and the numbers of PECs, neutrophils and macrophages were all decreased in acetate-treated mice during MSU- or alum-induced peritonitis (Fig. 3a–h). Moreover, acetate also suppressed the secretion of IL-6 and TNF-α in serum and IL-1β in serum and PLF during LPS-induced peritonitis (Fig. 3i–l). Collectively, these results demonstrated that acetate could suppress inflammation in vivo and attenuate NLRP3-mediated inflammasome activation in vitro and in vivo.
a–h Acetate (20 mM) suppressed MSU- (a–d) and alum-(e–g)-induced peritoneal inflammation. Flow cytometry revealed that neutrophils (CD11b+ GR-1+) (a, e), macrophages (F4/80+) (b, f), peritoneal exudate cells (PECs) (c, g) and IL-1β (ELISA) in the peritoneal lavage fluid (PLF) (d, h) were decreased in the acetate pretreatment group; i–l acetate suppressed LPS-induced peritonitis (n = 3). Serum levels of IL-6 (i), TNF-α (j), IL-1β (l), and IL-1β in PLF (k), as measured by ELISA, decreased in the acetate treatment group (n = 3); *P < 0.05, Student’s t-test compared with the model group (MSU, Alum or LPS). n = biological replicates. Data are presented as the mean ± SEM
Acetate-induced attenuation of the NLRP3 inflammasome is mediated by GPR43-Gq/11-Ca2+
We next analyzed the possible mechanism by which acetate mediates inflammasome suppression. Acetate has been proven to be capable of activating short-chain fatty acid receptors, including GPR41 and GRP4315. Previous studies have reported the anti-inflammatory effects of GPR43/41 in several biological processes, and among short-chain fatty acids, acetate is one of the strongest agonists of GPR4315. Therefore, we hypothesized that acetate exerts its inhibitory effect by activating a short-chain fatty acid receptor. To validate this hypothesis, we knocked down Gpr43 or Gpr41 in BMDMs (Supplemental Fig. 4A, B) and treated the cells with acetate before nigericin stimulation. The results showed that the knockdown of Gpr43 reversed the effect of acetate on the inflammasome, while the knockdown of GPR41 had no effect (Fig. 4a). We further used an agonist of GPR43 in BMDMs to validate the suppression of IL-1β and IL-18 in the supernatant. We found that the GPR43 agonist had an inhibitory effect similar to that of acetate (Fig. 4b), suggesting that acetate inhibited the inflammasome via GPR43 signaling.
a, b Acetate-induced suppression of the NLRP3 inflammasome is dependent on GPR43. ELISA of siRNA interference for GPR43 but not GPR41 (a) in LPS-primed BMDMs pretreated with acetate for 30 min (n = 6). ELISA showing the effect of a GPR43 agonist on IL-1β production (b) (n = 3). c, d Acetate-induced suppression of the NLRP3 inflammasome requires the Gq/11 subunit rather than the GI/O subunit. ELISA of IL-1β in GI/O subunit inhibitor-treated (c) and Gq/11 subunit siRNA knockdown (d) LPS-primed BMDMs (n = 6). e, f Fluorescence change of Fmax and F0 and representative curve of calcium changes in PMs with or without treatment with 2-APB (n = 4). g Chelation of Ca2+ (BAPTA) reversed the suppressive effects of acetate on IL-1β production (n = 3). h cAMP levels measured by ELISA were decreased by acetate in LPS + Nig-treated BMDMs (n = 3). n = biological replicates. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, Student’s t-test compared with the control group
Then, we asked why GPR43, but not GPR41, was required because acetate has an affinity for both receptors. Previous studies discovered that GPR41 couples to the GI/O subunit while GPR43 couples to either the GI/O or Gq/11 subunit16. We hypothesized that different G-protein subunits may enable GPR43 to perform distinct biological functions. Therefore, we used pharmacological and genetic methods to determine which subunit was involved in the inhibitory effect of acetate on the NLRP3 inflammasome. We found that treating BMDMs with pertussis toxin (PTX), a GI/O subunit inhibitor, did not alter the inhibitory effect of acetate, whereas knockdown of the Gq/11 subunit (Supplemental Fig. 4C) abolished the suppression of IL-1β secretion, suggesting that the Gq/11 subunit is essential for the effect of acetate (Fig. 4c, d).
Previous research reported that the Gq/11 subunit could couple with phospholipase C (PLC)19,20. This interaction may enable GPR43 to engage phosphatidylinositol signals when bound to acetate and subsequently lead to the hydrolysis of phosphatidylinositol bisphosphate (PIP2) to inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG); these products activate protein kinase C (PKC) and InsP3R, increasing the concentration of intracellular calcium and eventually promoting NLRP3 inflammasome activation8,21. Thus, we analyzed the changes in Ca2+ during acetate treatment. Using the Fluo 4-AM cytosolic calcium indicator, we found that acetate decreased cytosolic calcium levels after stimulation with nigericin, and this attenuation of nigericin-induced Ca2+ mobilization by acetate required InP3R participation, as the effect was reversed when InP3R signaling was inhibited by 2-APB (Fig. 4e, f and Supplemental Videos 1–4).
Next, we determined whether Ca2+ is required for these effects. Using BAPTA, a specific chelator of Ca2+, we found that the inhibitory effect of acetate on NLRP3 inflammasome activation was abrogated, suggesting that Ca2+ is essential for the mechanism by which acetate affects the NLRP3 inflammasome (Fig. 4g). Collectively, these results suggest that acetate prevents NLRP3 inflammasome activation via GPR43-Gq/11-Ca2+ signaling.
Acetate-mediated attenuation of the NLRP3 inflammasome depends on sAC-PKA signaling
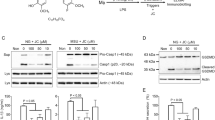
We next investigated the mechanism of GPR43-Gq/11-Ca2+ signaling in the prevention of NLRP3 inflammasome activation. cAMP, which is generated by adenylyl cyclase, is known to serve as a brake for inflammasome activation. However, the total intracellular cAMP level was reduced during acetate treatment (Fig. 4h). Thus, we further investigated the possible underlying mechanism of NLRP3 inflammasome attenuation. Soluble adenylyl cyclase (sAC) is distributed throughout the cell due to its structure, which is distinct from that of tmAC22,23,24,25, and sAC can be activated by Ca2+ to further activate downstream signaling pathways26,27. Therefore, we determined whether sAC is essential for NLRP3 inflammasome depression by acetate. First, we found that treatment with KH7, a sAC and tmAC inhibitor5,6,28, reversed the inhibitory effect of acetate, whereas treating BMDMs with KH7 alone did not induce IL-1β secretion (Fig. 5a). Second, consistent with previous data, acetate failed to inhibit IL-1β secretion when sAC was knocked down (Supplemental Fig. 4D and Fig. 5b). These results suggest that acetate may exert anti-inflammatory effects through interactions with sAC.
a, b ELISA of IL-1β indicated that acetate (20 mM) suppression in BMDMs was dependent on sAC. Inhibition of sAC (KH7) (a) or siRNA interference for two types of sAC (b) reversed the suppressive effects of acetate (n = 6). c ELISA of IL-1β indicated that the inhibition of PKA (H89) reversed the suppressive effects of acetate in BMDMs (n = 3). d Immunoprecipitation of NLRP3 polyubiquitination. The K48 and K63 ubiquitin chains were enhanced after acetate treatment (n = 3). e ELISA of IL-1β indicated that siRNA interference of MARCH7, an E3 ligase involved in NLRP3 ubiquitination, reversed the suppressive effects of acetate in BMDMs (n = 6). n = biological replicates. Data are presented as the mean ± SEM. *P < 0.05 and **P < 0.01, Student’s t-test compared with the control group
sAC converts ATP into cyclic AMP and consequently activates protein kinase A (PKA). Therefore, we investigated the role of PKA in acetate-induced NLRP3 inflammasome inhibition. H89, a selective inhibitor of PKA, was applied to LPS-primed BMDMs before acetate treatment. The results showed that H89 blocked acetate-induced inflammasome inhibition (Fig. 5c). Collectively, our data demonstrate that the inhibitory effect induced by acetate occurs in a sAC-PKA-dependent manner.
Acetate induces NLRP3 inflammasome polyubiquitination and autophagy
The relationship between ubiquitination and GPCR signaling has been investigated previously29, and growing evidence suggests that ubiquitination links GPCR signaling and inflammasome regulation5,6,8. Thus, to determine the role of GPCR signaling-driven ubiquitination in our study, we assessed the ubiquitination level of the NLRP3 inflammasome in BMDMs after treatment with acetate. We found that acetate promoted polyubiquitination of the NLRP3 inflammasome, which contained a mix of K48 and K63 ubiquitin chains (Fig. 5d). In addition, we found that RNA interference of MARCH7 (Supplemental Fig. 4E), a reported E3 ligase that promotes NLRP3 ubiquitination, led to the reversal of the acetate-induced IL-1β reduction (Fig. 5e). These results indicate that NLRP3 inflammasome polyubiquitination could be an essential event for the acetate-mediated suppression of NLRP3 inflammasome activation.
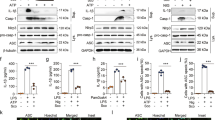
We further investigated whether autophagy or the proteasome participated in ubiquitination and protein degradation of the NLRP3 inflammasome. The results showed that both 3-MA and bafilomycin A could abolish the preventive effect of acetate on IL-1β secretion, while MG-132 could not (Fig. 6a–c), suggesting that autophagy, rather than proteasome-mediated proteolysis, was involved in acetate-induced NLRP3 inflammasome inhibition. We further measured NLRP3 and ASC in LPS-primed macrophages treated with acetate. We found that acetate promoted NLRP3 degradation but did not affect ASC, and this alteration was consistent with the changes in LC3B and p62 (Fig. 6c, d). In addition, after treatment with bafilomycin A1, which inhibits autophagy, we found that acetate increased the accumulation of LC3B, which indicates that acetate increased the formation of autophagosomes in a dose-dependent manner (Fig. 7a, b). These results suggest that acetate promotes degradation of the NLRP3 inflammasome by ubiquitination and subsequent autophagy.
a–c ELISA of IL-1β indicated that acetate (25, 30, and 35 mM) mediated suppression of the NLRP3 inflammasome in a manner dependent on autophagy but not the proteasome. Inhibition of autophagy (3-MA and bafilomycin A1) reversed the suppression of IL-1β (a, b), whereas inhibition of the proteasome induced few alterations (c) in BMDMs (n = 3). c, d Western blot of NLRP3, ASC, p62, and LC3B. The degradation of NLRP3 was accompanied by increased NLRP3 expression and decreased p62 expression in BMDMs (n = 3). n = biological replicates. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, Student’s t-test compared with the control group (a–c) or one-way ANOVA for comparisons of differences (d)
Discussion
Free fatty acids (FFAs), a diverse class of aliphatic hydrocarbon chains with a carboxyl group at one end, have been reported to participate in numerous biological processes, including immune regulation30. Different FFAs play distinct roles; for example, palmitate promotes IL-1β and IL-18 secretion in a dose-dependent manner7, whereas omega-3 fatty acids can inhibit the NLRP3 inflammasome via GPR120 and GPR4031. Very recently, the role of acetate in type 2 diabetes, heart failure and tumors has also been highlighted7,9,12. Acetate is present at a relatively low concentration in the blood32, but under specific conditions, acetate concentrations can increase to millimolar levels and exert a number of effects9,10,12. In vitro studies focusing on the effects of acetate at the cellular and molecular levels have generally applied a 10–20 mM concentration to mimic the effects of acetate in the microenvironment11,13. In the present study, we found that the anti-inflammatory effects of acetate were dose-dependent with no significant cytotoxicity, which is consistent with the results of previous research11.
Acetate shows a similar affinity for GPR41 and GRP4315. The present study showed that the acetate-mediated suppression of NLRP3 inflammasome activation relied on GPR43. We believe this effect may be due to the coupling GPRs to different signaling subunits. GPR43 exhibits dual coupling to the GI/O subunit and the Gq/11 subunit but not to the GS subunit4. Specific activation of GPR43 subsequently influences cAMP and Ca2+ and thus activates downstream signaling4, and the Gq/11 subunit is capable of activating PLC and IP3 to activate downstream signaling pathways20,33,34. Consistent with these conclusions, our results proved that acetate could decrease Ca2+ levels after stimulation and that this process required IP3R participation, as the blockage of IP3R reversed the Ca2+ decrease after acetate treatment. Moreover, although acetate decreased Ca2+ levels, the signal still required Ca2+ participation because the chelation of calcium also abolished the acetate-mediated attenuation of IL-1β. These results indicate that Gq/11-Ca2+ signaling is a possible mechanism for the acetate-mediated attenuation of the NLRP3 inflammasome and highlight the dual role of Ca2+ in signal transduction.
However, studies of calcium-sensing receptor (CaSR) found that increased Ca2+, accompanied by decreased cAMP, could further promote NLRP3 inflammasome activation8, which is inconsistent with the present finding. To address this issue, we focused on the crucial role of cAMP in inflammasome regulation. In recent years, several works found that cAMP could serve as a brake for the NLRP3 inflammasome. Dopamine and bile acid suppress the NLRP3 inflammasome via DR1 signaling or TGR5 signaling5,6 and consequently enhance the production of cAMP. The enhanced cAMP can further bind to NLRP3 directly to induce the ubiquitination of NLRP3 through PKA and thus suppress inflammasome activation5,6. Thus, the balance of Ca2+ and cAMP signaling determines the exact outcome of inflammasome activation35. cAMP is generated by two types of adenylyl cyclases that are present in different cellular compartments: the transmembrane adenylyl cyclase (tmAC) within the plasmalemma, and the soluble adenylyl cyclase (sAC) in the cytosol and within distinct organelles36,37,38,39. Unlike tmAC, sAC can be activated by Ca2+, generates cAMP40 and may mediate NLRP3 inflammasome attenuation. Indeed, although cAMP concentrations remain relatively low during acetate treatment, we still found an essential role for sAC in the acetate-mediated attenuation of IL-1β production, as both the KH7 inhibitor and siRNA interference of sAC reversed acetate-induced IL-1β attenuation. Hence, the present finding may raise the question of how the cell coordinates the convergence between two different sources of cAMP in regulating the ubiquitination of the NLRP3 inflammasome. Previous studies focused on the regulatory effect of cAMP produced by tmAC on NLRP3 degradation5,6,41, but few studies have focused on cAMP synthesized by sAC. In fact, the cAMP signal generated by tmAC is thought to be confined to defined compartments by PDE activity, which makes it difficult for the cAMP signal to be transmitted throughout the cytosol39. Considering the location of the NLRP3 receptor, sAC may take advantage of its location in interacting with the NLRP3 inflammasome. Thus, the present study indicates that compartmentalized cAMP, rather than total cAMP in the cytoplasm, may serve as a crucial factor in acetate-mediated attenuation of the NLRP3 inflammasome, supplementing the current theory of Ca2+/cAMP-mediated NLRP3 inflammasome attenuation.
Post translational modification has emerged as an endogenous regulatory mechanism of the inflammasome42, and several investigations have reported that ubiquitination acts as a negative regulator of NLRP3 inflammasome activation in a cAMP-PKA-dependent manner6,41. In this study, a similar pathway of PKA was also proven to be essential in acetate-induced ubiquitination of the NLRP3 inflammasome, and acetate subsequently induced inflammasome degradation through autophagy, rather than the proteasome. Thus, the present study suggests that acetate may bind to GPR43 and signal through sAC-PKA to attenuate the inflammasome.
Our data also showed that acetate plays a protective role in vivo. A previous study found that suppressing the NLRP3 inflammasome by blocking PKM2-dependent glycolysis protects mice from endotoxemia and polymicrobial sepsis43. This protective effect of acetate may be consistent. On the one hand, NLRP3 is reported to be involved in endotoxemia44 and acetate attenuates NLRP activation. On the other hand, our data also suggest that acetate somewhat attenuates LPS-induced NF-κB signaling activation (Fig. S2). Thus, by showing the protective effects of acetate in LPS-induced endotoxemia and the NLRP3 inflammasome-dependent peritonitis model, the present study may serve as the basis for more extensive research on acetate in sepsis and other inflammatory diseases.
In summary, in the present study, we demonstrate that acetate and GPR43 signaling attenuate NLRP3 inflammasome activation, and we report a novel mechanism of inflammasome regulation by metabolites. Additionally, our results reveal a critical role for sAC in NLRP3 inflammasome activation, which supplements the present mechanism of NLRP3 inflammasome regulation. The regulatory effect of acetate on inflammation provides an example of the mechanisms by which metabolites regulate immune responses and may offer a new therapeutic strategy for the clinical management of inflammatory diseases.
Change history
14 August 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426 (2002).
Wen, H., Miao, E. A. & Ting, J. P. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity 39, 432–441 (2013).
Guarda, G. et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 (2011).
Thorburn, A. N., Macia, L. & Mackay, C. R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 40, 833–842 (2014).
Guo, C. et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity 45, 802–816 (2016).
Yan, Y. et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160, 62–73 (2015).
Wen, H. et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415 (2011).
Lee, G. S. et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2 + and cAMP. Nature 492, 123–127 (2012).
Gao, X. et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat. Commun. 7, 11960 (2016).
Perry, R. J. et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534, 213–217 (2016).
Bolduc, J. F., Hany, L., Barat, C., Ouellet, M. & Tremblay, M. J. Epigenetic metabolite acetate inhibits class I/II histone deacetylases, promotes histone acetylation, and increases HIV-1 integration in CD4(+) T cells. J. Virol. 91, pii: e01943–16 (2017).
Marques, F. Z. et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135, 964–977 (2017).
Macia, L. et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 6, 6734 (2015).
Le Poul, E. et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489 (2003).
Brown, A. J. et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319 (2003).
Maslowski, K. M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009).
Latz, E., Xiao, T. S. & Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Chae, J. J. et al. Connecting two pathways through Ca 2 + signaling: NLRP3 inflammasome activation induced by a hypermorphic PLCG2 mutation. Arthritis Rheuma. 67, 563–567 (2015).
Wang, B. et al. Muscarinic suppression of ATP-sensitive K(+) channels mediated by the M3/Gq/11/phospholipase C pathway contributes to mouse ileal smooth muscle contractions. Am. J. Physiol. Gastrointest. Liver Physiol. 12, G618–G630 (2018).
Mulay, S. R. et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1beta secretion. J. Clin. Invest. 123, 236–246 (2013).
Han, H. et al. Calcium-sensing soluble adenylyl cyclase mediates TNF signal transduction in human neutrophils. J. Exp. Med. 202, 353–361 (2005).
Hess, K. C. et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell. 9, 249–259 (2005).
Wu, K. Y. et al. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat. Neurosci. 9, 1257–1264 (2006).
Dessauer, C. W. et al. International union of basic and clinical pharmacology. CI. structures and small molecule modulators of mammalian adenylyl cyclases. Pharm. Rev. 69, 93–139 (2017).
Steegborn, C. Structure, mechanism, and regulation of soluble adenylyl cyclases—similarities and differences to transmembrane adenylyl cyclases. Biochim. Biophys. Acta 1842, 2535–2547 (2014).
Inda, C. et al. Different cAMP sources are critically involved in G protein-coupled receptor CRHR1 signaling. J. Cell Biol. 214, 181–195 (2016).
Bitterman, J. L., Ramos-Espiritu, L., Diaz, A., Levin, L. R. & Buck, J. Pharmacological distinction between soluble and transmembrane adenylyl cyclases. J. Pharm. Exp. Ther. 347, 589–598 (2013).
Wojcikiewicz, R. J. Regulated ubiquitination of proteins in GPCR-initiated signaling pathways. Trends Pharm. Sci. 25, 35–41 (2004).
Alvarez-Curto, E. & Milligan, G. Metabolism meets immunity: the role of free fatty acid receptors in the immune system. Biochem. Pharmacol. 114, 3–13 (2016).
Yan, Y. et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 38, 1154–1163 (2013).
Wolin, M. J. & Miller, T. L. Interactions of microbial populations in cellulose fermentation. Fed. Proc. 42, 109–113 (1983).
Qian, J. et al. Agonist-induced activation of human FFA1 receptor signals to extracellular signal-regulated kinase 1 and 2 through Gq- and Gi-coupled signaling cascades. Cell Mol. Biol. Lett. 22, 13 (2017).
Zulfiker, A. H., Hashimi, S. M., Good, D. A., Grice, I. D. & Wei, M. Q. Cane toad skin extract-induced upregulation and increased interaction of serotonin 2A and D2 receptors via Gq/11 signaling pathway in CLU213 cells. J. Cell Biochem. 118, 979–993 (2017).
Horng, T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol. 35, 253–261 (2014).
Tresguerres, M., Levin, L. R. & Buck, J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int. 79, 1277–1288 (2011).
Schmid, A., Meili, D. & Salathe, M. Soluble adenylyl cyclase in health and disease. Biochim. Biophys. Acta 1842, 2584–2592 (2014).
Valsecchi, F., Konrad, C. & Manfredi, G. Role of soluble adenylyl cyclase in mitochondria. Biochim. Biophys. Acta 1842, 2555–2560 (2014).
Ladilov, Y. & Appukuttan, A. Role of soluble adenylyl cyclase in cell death and growth. Biochim. Biophys. Acta 1842, 2646–2655 (2014).
Zippin, J. H. et al. CO2/HCO3− and calcium-regulated soluble adenylyl cyclase as a physiological ATP sensor. J. Biol. Chem. 288, 33283–33291 (2013).
Han, S. et al. Lipopolysaccharide primes the NALP3 inflammasome by inhibiting its ubiquitination and degradation mediated by the SCFFBXL2 E3 ligase. J. Biol. Chem. 290, 18124–18133 (2015).
Yang, J., Liu, Z. & Xiao, T. S. Post-translational regulation of inflammasomes. Cell Mol. Immunol. 14, 65–79 (2017).
Xie, M. et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat. Commun. 7, 13280 (2016).
Lamkanfi, M. et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 185, 4385–4392 (2010).
Acknowledgements
This research was funded by the National Natural Science Foundation of China (81772105 to X.M.D., 31370865 and 81771697 to Y.Z., 81871579 and 81671939 to J.J.B., 81600948 to Y.P.Z., and 81671887 to L.L.B.), the Shanghai Outstanding Youth Medical Professionals Training Program (No. 2017YQ015 to L.L.B.) and the Scientific Research Foundation of Shanghai Municipal Commission of Health and Family Planning (201740050 to N.L.). We would like to thank Mr. Jun Wang and Mrs. Kangqing Fang for technical support and kind help during the experiments. We appreciate the careful animal care work of Mrs. Guiying Ji.
Authors' contributions
M.D.X. performed most of the experiments. Z.Y.J. performed some of the experiments and prepared the manuscript. C.L.W. and N.L. offered technical support and helped with some experiments. L.L.B., Y.P.Z. and J.J.B. provided constructive suggestions and discussions during the study. Y.Z. and X.M.D. designed the entire study and secured funding for the research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, M., Jiang, Z., Wang, C. et al. Acetate attenuates inflammasome activation through GPR43-mediated Ca2+-dependent NLRP3 ubiquitination. Exp Mol Med 51, 1–13 (2019). https://doi.org/10.1038/s12276-019-0276-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-019-0276-5
This article is cited by
-
Limosilactobacillus fermentum Strains as Novel Probiotic Candidates to Promote Host Health Benefits and Development of Biotherapeutics: A Comprehensive Review
Probiotics and Antimicrobial Proteins (2024)
-
Host microbiome associated low intestinal acetate correlates with progressive NLRP3-dependent hepatic-immunotoxicity in early life microcystin-LR exposure
BMC Pharmacology and Toxicology (2023)
-
Serum metabolomic profiling identifies potential biomarkers in arthritis in older adults: an exploratory study
Metabolomics (2023)
-
Limosilactobacillus fermentum Strains with Claimed Probiotic Properties Exert Anti-oxidant and Anti-inflammatory Properties and Prevent Cardiometabolic Disorder in Female Rats Fed a High-Fat Diet
Probiotics and Antimicrobial Proteins (2023)
-
Propionate and butyrate attenuate macrophage pyroptosis and osteoclastogenesis induced by CoCrMo alloy particles
Military Medical Research (2022)