Abstract
Cancer immunotherapy is a promising way to eliminate tumor cells by using the patient’s own immune system. Selecting the appropriate animal models to develop or validate preclinical immunotherapeutic trials is now an important aspect of many cancer research programs. Here we discuss the advantages and limitations of using genetically engineered immunodeficient mouse models, patient-derived xenografts (PDXs), and humanized mouse models for developing and testing immunotherapeutic strategies.
Similar content being viewed by others
Introduction
Immune surveillance against cancer is an important protection of the host to restrain carcinogenesis and sustain cellular homeostasis. Immune surveillance has three essential phases: elimination, equilibrium, and escape1. During the interaction of host cells with tumor cells, the immune system is usually capable of recognizing tumor cells from novel cell surface antigens that arise from genetic and/or epigenetic changes in the tumor cells. To overcome this immune surveillance, tumor cells can use a variety of mediators for immunosuppression. For example, T-cell activation can be suppressed by adenosine and vascular endothelial growth factor A, which are released by tumor cells under hypoxia. Another mechanism for immune suppression is when tumor cells downregulate major histocompatibility complex (MHC) class I (reducing the presentation of intracellular peptide fragments on the cell surfaces of tumor cells) or disable other components of the antigen-presenting process, to escape T-cell recognition2, 3.
The concept of cancer immunotherapy was initiated by William B. Coley, who observed tumor shrinkage and disappearance following treatment with a bacterial toxin in the 1890s. Since then, immunotherapy has subsequently developed into a novel method for treating cancer by reinforcing the immune system, rather than attacking the tumor cells directly with chemotherapeutic agents. Immunotherapy treatment can be broadly classified as either cancer vaccines, adoptive cellular immunotherapy, or therapies using immune checkpoint blockades4.
To perform systematic preclinical cancer immunotherapy studies, it is important to select appropriate animal models. Various animal models, namely, genetically engineered mice, patient-derived xenografts (PDXs), and humanized PDX mouse models, can be used for testing new anticancer immunotherapies. In this review, we discuss the basic principle of PDXs and humanized mouse models and their applications in cancer research. We also discuss the limitations of these models and review strategies that may be used to overcome these limitations.
Cancer immunotherapy
The host immune system has an important role in tumor development and control. Cancer immunotherapy uses the immune system to eliminate tumor cells. Currently, there are various approaches to cancer immunotherapy, including cancer vaccines, adoptive cell therapies (ACT) and immune checkpoint blockade therapies (Table 1).
Cancer vaccines
Cancer vaccines can be classified into the following two categories: prophylactic and therapeutic5. Prophylactic vaccines have been used with considerable success for the prevention of cancers of viral origin such as for cervical and liver cancers6, 7. Therapeutic vaccines have been developed to specifically stimulate CD8+ and CD4+ T cells. These vaccines can then target the differentiated antigens expressed on the cell surfaces of tumor cells8. However, cancer vaccines, consisting of short peptides, can be rapidly cleared before being loaded onto antigen-presenting cells (APCs). In other cases, the therapeutic benefits can be limited by an insufficient immunization or response to a selected tumor antigen3. Finally, these vaccines require knowledge and purification of tumor-specific antigens. Since this is still quite limited, this technology is currently applicable to only a few types of cancers and stages9.
Dendritic cells (DCs) have been shown to be more efficient at antigen presentation and the induction of T-cell immunity compared to other APCs such as macrophages. In this approach, DCs are isolated from the patient’s peripheral blood mononuclear cells (PBMC), loaded with tumor antigens ex vivo, activated, and then reinfused back into the patient10, 11. Indeed, DC vaccinations have already produced some meaningful clinical results in a subset of patients with advanced cancers12. For instance, treatment with sipuleucel-T (a cellular product based on enriched blood APCs briefly cultured with a prostatic acid phosphatase fusion protein) achieved an approximate four-month improvement in the median survival for some patients with metastatic prostate cancer13, 14.
Adoptive cell therapy
The treatment of cancer patients with immune cells isolated from the body, expanded ex vivo, and reinjected into the patient for tumor cell targeting that ultimately induces cell death is called ACT. In the host, T-cell-mediated antitumor immune responses can be triggered by APCs that capture antigens from tumor cells. T cells then scan for unrecognized MHC-peptide complexes, which would alert them to potentially threatening foreign antigens and then lead to the activation of their T-cell receptors15. Tumor cells generally express antigens that are characteristic of their tissues of origin, and tissue-differentiated antigens are attractive targets for ACT.
For immunotherapies based on the adoptive transfer of tumor infiltrated lymphocytes (TILs), genetically engineered T cells that express T-cell receptors (TCRs) with a high affinity and specificity for target antigens would be an excellent treatment option16,17,18,19,20. Chimeric antigen receptors (CARs) are one way for providing specificity to transduced T cells (CAR-T cells) and can originate from antibodies. CARs recognize MHC-nonrestricted structures on the surfaces of target cells, whereas TCRs recognize mainly intracellular antigens that have been presented as peptide complexes with MHC molecules21, 22. The genetic modification of T cells for ACT can confer new antigenicity to recipient T cells. However, there are several limitations to this method. For example, current approaches have only monoclonal specificity and may only be effective for the treatment of a small proportion of the tumor cells. In the case of genetically engineered TCRs, mispairing of TCRs with endogenous TCR chains can also occur23.
Immune checkpoint blockade therapy
Immune checkpoints are regulators of the immune system and play an important role in preventing self-attacks by the immune system24. Malignant cells usually express unique antigens, which allow our immune system to differentiate them from our normal cells and subsequently remove them. However, many tumor cells have evolved a mechanism to escape from the host immune system, by expressing cell surface molecules, which interact with immune checkpoint receptors on T cells causing the T cells to erroneously classify the tumor cells as healthy normal cells. Hence, inhibiting these checkpoint receptors (on either the host T cells or the tumor cells) can potentially be an effective means for allowing the host T cells to again properly classify tumor cells. Well-known immune checkpoint inhibitors include those that target programmed cell death protein 1 (PD-1, also known as CD279) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4, also known as CD152) (Fig. 1a, b)25, 26. Already, a significant number of clinical studies have been conducted using this strategy for cancer therapy and have demonstrated efficacy of immune checkpoint blockades in a variety of solid tumors and hematologic malignancies27.
a Programmed cell death protein-1 (PD-1) inhibits the activation and function of T cells. When PD-1 on activated T cells binds to programmed cell death ligand 1 (PD-L1) or programmed cell death ligand 2 (PD-L2) on tumor cells, T cells become inactivated, allowing tumor cells to evade the host immune response. Anti-PD-1 or PD-L1 antibodies can inhibit suppressive effects of tumor cells and enhance antitumor activities. b T-cell activation requires both TCR signaling and co-stimulatory signaling (CD28). When T cells get activated, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is upregulated and displaced by CD28 binding to B7 due to higher binding affinity. The binding of B7 proteins to CTLA-4 inhibits T-cell activation
Overview of PDX models
In vitro methods for testing anticancer drugs can employ monolayer cell cultures or organoid cultures and are especially beneficial in genetic modification and high-throughput screening assays. However, these methods have some limitations such as the selective proliferation of clonal cells28. PDX models are thought to overcome some of these limitations and seem to preserve key characteristics of the patient’s tumors, including histological features, genomic signatures, and the genetic heterogeneity of cells in a tumor mass29. Therefore, they seem to recapitulate many aspects of the original patient tumor and can potentially serve as a more accurate predictive platform for therapeutic outcomes30.
Generation of the PDX models
PDX models are generated by the implantation of fresh human tumor tissues into immunodeficient mice, to reduce rejection of the tumor cells by the mouse. Tumor tissues, no larger than 2 mm3, are implanted into immunodeficient mice either subcutaneously or orthotopically (i.e., at the same tissue-of-origin site). One or two fragments are generally implanted into each mouse. The tumor masses are then grown to ~1000 mm3 in size, at which point, the tumors can be cryopreserved, characterized, or dissected again for reimplantation and propagation in additional mice (Fig. 2). For hematological cancers, PBMCs or bone marrow samples from patients with leukemia are engrafted either in the blood stream or into the bone marrow31, 32. The continued propagation of the human tumors in the immunodeficient mice make PDX models a renewable resource for cancer studies.
Tumor tissues from cancer patients are implanted into immunodeficient mice (P0) subcutaneously or orthotopically. After the growth of tumor tissues in P0 mice, these tissues are used for genomic analysis such as whole exome sequencing (WES), RNA sequencing (RNA-seq) and copy number alteration (CNA) analysis and can be preserved or reimplanted into new mice for tumor cell passaging. After more expanding of these tumor xenografts, in vivo drug response testing can be performed in these models
There are a variety of immunodeficient mice that can be used for PDX models: severely compromised immune-deficient (SCID) mice, athymic nude mice, non-obese diabetic (NOD)–SCID mice, and recombination-activating gene 2 (Rag2)-knockout mice33. More recently, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ(NSG) mice have become the mouse strain of choice for such PDX studies because this mouse has no IL-2 receptor gamma, which is an important component of the surface receptor of immune cells that transduce signals from six kinds of interleukins. Since the signaling pathway of IL-2 receptor gamma is needed for the differentiation and function of many hematopoietic cells, the absence of this receptor leads to a dysfunction in innate immunity, including natural killer (NK) cells. This makes the NSG mouse a very effective model for the engraftment of primary tumor tissues or cells34, 35.
Applications of PDX models in cancer research
PDX models for exploring drug efficacy and the mechanism of resistance
PDX models have already been used in a diverse range of preclinical cancer research projects. For example, a recent study using PDXs demonstrated the inhibition of the nuclear exporter for antitumor efficacy in a triple-negative breast cancer36. In another study, lung cancer PDX models revealed the antitumor activity of kinetochore-associated protein 2 siRNA37. Similarly, another study demonstrated the antitumor effects of multikinase inhibitors in PDX models of hepatocellular carcinoma38.
One problem that continues to be explored is how well the drug efficacy data using these PDX models correlate with actual clinical outcomes39. Several studies have been published that seem to suggest that in some cases, there can be good correlations between the two. For example, one study showed a high correlation between PDX models and clinical trials for over 3300 drug response datasets40. In another study, PDX models of colorectal cancers treated with an epidermal growth factor receptor inhibitor, cetuximab, showed comparable response rates to those of the patients in whom the tumor orginated41. Finally, responses to sirolimus, sunitinib, and dovitinib, but not erlotinib, were largely correlated between PDX models and corresponding clinical outcome results for renal cell cancers42.
Having a good correlation between PDX models and clinical trials provides a chance to find novel biomarkers for drug reactivity. For example, in a melanoma PDX model introducing vemurafenib resistance, the resistant tumors showed dependency on BRAF signaling due to the elevated BRAF (V600E) expression43. These data suggest the possibility that elevated BRAF expression could be used as a biomarker for vemurafenib resistance43. Another study found a molecular mechanism for gemcitabine resistance through the use of PDX models for pancreatic cancer44.
PDX models for co-clinical trials
Co-clinical trials are preclinical research studies that can be conducted in parallel with human patient treatments in the clinical setting. In this application, PDX models are generated from cancer tissues of patients in clinical trials, and the PDXs are treated with the same and possibly additional therapies to follow clinical responses45. The responses to new agents, mechanisms underlying the responses to treatment, and explorations of prognostic biomarkers can be studied by using established PDXs from patients being treated. Using such co-clinical trials, strategies for new combinations can also be suggested. For example, a phase II co-clinical trial of arsenic trioxide in relapsed small cell lung cancer revealed that PDX modeling reliably reproduced clinical outcomes46. Another recent study revealed that the response to dovitinib in lung squamous cell carcinomas could be predicted by signatures of FGFR gene expression47. Such co-clinical trials provide a chance to evaluate the efficacies of several drugs, in isolation or combination, in a cost-effective and cost-efficient manner.
Limitations of PDX Models
Although PDX models are excellent in vivo platforms for precision medicine, they have several limitations that should be noted. First, the development of a PDX model from a patient can take as long as 6 months (or longer) to establish. In addition, certain tumor types, such as prostate cancers, are difficult to establish as PDX models, presumably because of the need for additional, unknown “factors” needed for proper tumor growth. Finally, those tumors that have genetic heterogeneity cannot always be recapitulated in serial passages if the genetic heterogeneity is not all represented in the dissected tumor that is passaged.
Humanized mice with PDXs: a groundbreaking research platform for cancer immunology
As outlined above, PDX models can be a useful resource for preclinical trials; however, these are limited to chemotherapeutic drugs. For immunotherapeutic options, an intact immune system is required. The use of mice with a murine immune system is limiting, as it does not accurately recapitulate the human immune system. Therefore, creating a mouse with a human immune system (a “humanized mouse”) is a better vehicle for immunotherapeutic efficacy testing.
Creating humanized mice: an in vivo model that uses human immune systems
The ultimate goal of humanization is to generate mice with a fully competent human immune system, capable of mounting anticancer immune responses for specific immunotherapeutic interventions (Fig. 3). In its most basic form, the NSG mouse can be engrafted with various types of human leukocytes and purified human CD34+ hematopoietic stem cells (HSCs), obtained from bone marrow, umbilical cord blood, fetal livers or thymus tissues48.
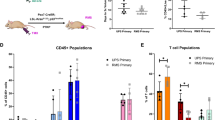
The NSG mice are irradiated with whole body gamma irradiation. Human CD34+ hematopoietic stem cells (HSCs) are intravascularly injected into NSG mice at 5 weeks. Humanization for the engraftment of human HSCs is monitored by flow cytometry of use for determining the percentage of differentiated human CD45+ cells in the peripheral blood of the mice. Patient tumor tissues are then engrafted into ‘humanized’ mice and used for studying the efficacy of variety therapies. These humanized PDX models can also permit the study of human immune responses, including the analysis of TILs, cytokines, and antibodies
Humanized PDX models are generated by implanting fresh human tumor fragments into these humanized mice. Initially, NSG mice that are 5 to 12 weeks old are first irradiated with 50 to 250 cGy whole body gamma irradiation to enhance engraftment. Then, 3 × 104–1 × 105 human CD34+ HSCs are intravascularly injected into each irradiated NSG mouse. At approximately 10–12 weeks of age, engraftment of the human HSCs can be confirmed by assessing for differentiated human CD45+ cells (leukocyte common antigen) in the peripheral blood of the mice using flow cytometry. Human CD45+ cells can be detected as early as 4 weeks after the engraftment of HSCs49. Successful engraftment of a human immune system can be considered when the mice have more than 25% human CD45+ cells in their peripheral blood. The process for generating humanized mice is summarized in Fig. 3. Specific PDXs can then be inserted into the humanized mice, and an immunotherapeutic agent is subsequently applied for testing. Afterward, the host immune response to an immunotherapeutic agent can be analyzed using different methods (Fig. 3).
To produce humanized mice effectively, several immunodeficient mouse strains have been developed. For example, the NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV IL-3, CSF2, KITLG)1Eav/MloySzJ (also known as NSG-SGM3) mice expresses human IL-3, Granulocyte-macrophage colony-stimulating factor (GM-CSF) and stem cell factor allows for the stable engraftment of human HSCs for humanization50. In addition, the NOD,B6.SCID Il2rγ−/− KitW41/W41 (NBSGW) mice, which carry mutations in c-Kit, support the transplantation of HSCs without irradiation51, 52. The human SIRPA and IL15 knockin (SRG-15) mice also showed an increased development of intraepithelial lymphocytes, innate lymphoid cell subsets and NK cells53.
Ideally, the humanized mice being used would have the same immune system from which the PDX was derived. However, it is very difficult to obtain CD34+ cells from the cancer patient, and therefore, an allogenic immune approach is usually used.
Application of humanized mice with PDXs for cancer research
The tumor microenvironment
The relationship between the tumor and the surrounding stromal and immune cells is highly complex and an area of exciting research54. Indeed, the interplay between the rapidly dividing cancer cells and the surrounding stromal tissue is thought to be one critical factor in treatment efficacy55. Cancer cells can interact with the stromal microenvironment in several ways. (1) As cancer cells divide, they can recruit cells that contribute to the infrastructure of the growing tumor, including fibroblasts, endothelial cells, and circulating immune cells56. Chemokines released from cancer cells, stromal cells and leukocytes can regulate angiogenesis. Cancer cells also produce cytokines (protein messengers that tell other cells when and where to launch an immune response) to control nearby immune cells and help them escape immune surveillance. Using humanized mice, Morton and colleagues reported that human immune cells maintained the microenvironment of engrafted cancer PDXs57. The human immune cells, which infiltrated the engrafted tumors, induce lymphangiogenesis and sustain the original gene expression profile of the PDX57. This study indicates that interactions between stromal immune cells and tumor cells are indeed important in maintaining the integrity of the original tumor.
Humanized mouse model for cancer immunotherapy
Numerous recent studies have now demonstrated the advantages and advancements of cancer immunotherapy using humanized mice. For example, in a recent study, a renal cell carcinoma (RCC) mouse model was generated orthotopically with PBMCs to evaluate the antibody efficacy targeting the carbonic anhydrate IX protein in RCC. This study demonstrated that the antibody inhibited cancer growth by priming T-cell activity58. In October 2017, Akiyama and colleagues demonstrated that the STAT3 inhibitor, STX-0119, had an antitumor activity, whereby lymphocytes accumulated within the engrafted tumor tissues of the humanized mice59. Similarly, another study demonstrated that a PD-1 inhibitor could effectively restrain osteosarcoma pulmonary metastasis60. Finally, Hu Z et al., collected T cells from humanized mice, and found these T cells to have cytotoxic activity to melanoma cells in vitro in an antigen-specific manner. The transfer of these T cells to a melanoma-bearing PDX model substantially extended the animal’s survival61. This study demonstrates that humanized mice are a useful resource to study the functions of antigen-specific T cells for cancer immunotherapy.
Humanized mouse models can also be used to study the complicated relationship between tumor development, oncolytic viruses, and the human immune system. Tsoneva and colleagues placed lung carcinomas into humanized mice to determine the interaction of the oncolytic vaccinia virus (VACV) with the host immune system and how it affects tumor growth62. They validated the efficacy of combination therapy using CTLA4-blocking antibody and VACV, which was only possible with the humanized mouse model62.
Humanized mouse models have also been used to study the efficacy of ACT treatments. For example, to study ACT and the immune checkpoint blockade, Jespersen and colleagues inserted melanoma tumor cells and T cells from the same patient into humanized mice and showed tumor inhibition63. Interestingly, T cells from ACT non-responders failed to inhibit tumor growth in the same model63. It would be important to explore more studies for many other types of well-characterized human cancers.
Human microbiota-associated mice
The microbiota comprises commensal and other microorganisms that inhabit the epithelial barriers of the host body64. The microbiota regulates many physiological functions in the host body, including metabolism, hematopoiesis and immunity65. The homeostatic interaction with these microorganisms is particularly important for the development of the host immune system65,66,67. However, commensal microorganisms exist in a balance that, if significantly altered, enters dysbiosis, which can be associated with the etiology of many types of cancers68,69,70,71,72. Indeed, recent studies have shown some evidence that the microbiota, especially within the gut, can impact the efficacy of certain cancer therapies, including chemotherapy, radiotherapy and immunotherapy73. Considering the importance of the microbiota, further studies of the effects of the microbiota on biological responses would be useful.
Because germ-free mice have no microbes, researchers have tried to control the microbiota through direct inoculation with a specific microorganism or combinations of them64. Therefore, researchers have developed human microbiota-associated mice that are established from germ-free mice by fecal microbiota transplantation74, 75. This method has been used to study the interaction between the host and microorganism as well as the effects of microbes on human health and diseases, including cancers64.
Future directions
In this review, we have shown many advantages for the use of humanized mice in studying cancers. In particular, there is an ongoing need to generate more comprehensive and functional immune systems within these humanized mice and identify new practical approaches that would enable autologous experiments that engraft diseased tissues and immune cells from the same individual for a more accurate understanding of disease progression and personalized treatment efficacy. Despite the noted limitations, the use of PDX models in humanized mice has already provided many insights into the behaviors of diverse cancers within their native tumor microenvironments, under the effects of human immune cells. As humanized PDX models continue to improve and truly recapitulate the human biological system, these models will provide an unprecedented research platform in cancer immunology and personalized medicine.
References
Kim, R., Emi, M. & Tanabe, K. Cancer immunoediting from immune surveillance to immune escape. Immunology 121, 1–14 (2007).
Shin, J. Y., Yoon, I. H., Kim, J. S., Kim, B. & Park, C. G. Vascular endothelial growth factor-induced chemotaxis and IL-10 from T cells. Cell. Immunol. 256, 72–78 (2009).
Mellman, I., Coukos, G. & Dranoff, G. Cancer immunotherapy comes of age. Nature 480, 480–489 (2011).
Oiseth, S. J. & Aziz, M. S. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J. Cancer Metastas-. Treat. 3, 250–261 (2017).
Palucka, K., Banchereau, J. & Mellman, I. Designing vaccines based on biology of human dendritic cell subsets. Immunity 33, 464–478 (2010).
Bayas, J. M., Costas, L. & Munoz, A. Cervical cancer vaccination indications, efficacy, and side effects. Gynecol. Oncol. 110, S11–S14 (2008).
MH, C. Hepatitis B Virus and cancer prevention. Clin. Cancer Prev. 188, 75–84 (2010).
Boon, T., Coulie, P. G., Van den Eynde, B. J. & van der Bruggen, P. Human T cell responses against melanoma. Annu. Rev. Immunol. 24, 175–208 (2006).
Guo, C. et al. Therapeutic cancer vaccines: past, present, and future. Adv. Cancer Res. 119, 421–475 (2013).
Schuler, G. Dendritic cells in cancer immunotherapy. Eur. J. Immunol. 40, 2123–2130 (2010).
Sabado, R. L. & Bhardwaj, N. Dendritic cell immunotherapy. Ann. N. Y. Acad. Sci. 1284, 31–45 (2013).
Boudewijns, S., Bloemendal, M., Gerritsen, W. R., de Vries, I. J. & Schreibelt, G. Dendritic cell vaccination in melanoma patients: From promising results to future perspectives. Hum. Vaccin. Immunother. 12, 2523–2528 (2016).
Higano, C. S. et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 115, 3670–3679 (2009).
Kantoff, P. W. et al. Sipuleucel-T Immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Restifo, N. P., Dudley, M. E. & Rosenberg, S. A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12, 269–281 (2012).
Brentjens, R. J. et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118, 4817–4828 (2011).
Porter, D. L., Levine, B. L., Kalos, M. & Bagg, A. & June, C. H. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Robbins, P. F. et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29, 917–924 (2011).
Kochenderfer, J. N. et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720 (2012).
Pule, M. A. et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 14, 1264–1270 (2008).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010).
Sadelain, M., Brentjens, R. & Riviere, I. The promise and potential pitfalls of chimeric antigen receptors. Curr. Opin. Immunol. 21, 215–223 (2009).
Koya, R. C. et al. Kinetic phases of distribution and tumor targeting by T cell receptor engineered lymphocytes inducing robust antitumor responses. Proc. Natl Acad. Sci. USA 107, 14286–14291 (2010).
Kim, J. M. & Chen, D. S. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann. Oncol. 27, 1492–1504 (2016).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Dammeijer, F., Lau, S. P., van Eijck, C. H. J., van der Burg, S. H. & Aerts, J. Rationally combining immunotherapies to improve efficacy of immune checkpoint blockade in solid tumors. Cytokine Growth Factor Rev. 36, 5–15 (2017).
Thallinger, C. et al. Review of cancer treatment with immune checkpoint inhibitors: Current concepts, expectations, limitations and pitfalls. Wien. Klin. Wochenschr. 130, 85–91 (2018).
Gao, D. & Chen, Y. Organoid development in cancer genome discovery. Curr. Opin. Genet. Dev. 30, 42–48 (2015).
Heid, I. et al. Co-clinical assessment of tumor cellularity in pancreatic cancer. Clin. Cancer Res. 23, 1461–1470 (2017).
Kopetz, S., Lemos, R. & Powis, G. The promise of patient-derived xenografts: the best laid plans of mice and men. Clin. Cancer Res. 18, 5160–5162 (2012).
Christie, A. L. et al. T-cell lymphoma patient-derived xenografts and newly developed cell lines recapitulate aspects of disease biology and represent novel tools for preclinical drug development. Blood 128, 3015 (2016).
Ye, W. et al. Quantitative evaluation of the immunodeficiency of a mouse strain by tumor engraftments. J. Hematol. Oncol. 8, 59 (2015).
Morton, C. L. & Houghton, P. J. Establishment of human tumor xenografts in immunodeficient mice. Nat. Protoc. 2, 247–250 (2007).
Shultz, L. D. et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174, 6477–6489 (2005).
Greiner, L., Hesselton, R. A. & Shultz, L. D. SCID mouse models of human stem cell engraftment. Stem Cells 16, 166–177 (1998).
Arango, N. P. et al. Selinexor (KPT-330) demonstrates anti-tumor efficacy in preclinical models of triple-negative breast cancer. Breast Cancer Res. 19, 93 (2017).
Makita, Y. et al. Antitumor activity of kinetochore-associated protein 2 siRNA against lung cancer patient-derived tumor xenografts. Oncol. Lett. 15, 4676–4682 (2018).
Kissel, M. et al. Antitumor effects of regorafenib and sorafenib in preclinical models of hepatocellular carcinoma. Oncotarget 8, 107096–107108 (2017).
J, D. et al. Validation of a preclinical model for assessment of drug efficacy in melanoma. Oncotarget 7, 13069–13081 (2016).
Gu, Z. et al. Evaluation of the correlations between patient-derived xenograft (PDX) model-based mouse trials and cancer patient-based clinical trials. J. Clin. Oncol. 35, e23140–e23140 (2017).
Bertotti, A. et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 1, 508–523 (2011).
Sivanand, S. et al. A validated tumorgraft model reveals activity of dovitinib against renal cell carcinoma. Sci. Transl. Med. 4, 137ra175 (2012).
Das Thakur, M. et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 494, 251–255 (2013).
Sebastiani, V. et al. Immunohistochemical and genetic evaluation of deoxycytidine kinase in pancreatic cancer: relationship to molecular mechanisms of gemcitabine resistance and survival. Clin. Cancer Res. 12, 2492–2497 (2006).
Hidalgo, M. et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 4, 998–1013 (2014).
Owonikoko, T. K. et al. Patient-derived xenografts faithfully replicated clinical outcome in a phase II co-clinical trial of arsenic trioxide in relapsed small cell lung cancer. J. Transl. Med. 14, 111 (2016).
Kim, H. R. et al. Co-clinical trials demonstrate predictive biomarkers for dovitinib, an FGFR inhibitor, in lung squamous cell carcinoma. Ann. Oncol. 28, 1250–1259 (2017).
Douglas, D. N. & Kneteman, N. M. Generation of improved mouse models for the study of hepatitis C virus. Eur. J. Pharmacol. 759, 313–325 (2015).
Pearson, T., Greiner, D. L. & Shultz, L. D. Creation of “humanized” mice to study human immunity. Curr. Protoc. Immunol. Chapter 15, Unit 15 21. https://doi.org/10.1002/0471142735.im1521s81 (2008).
Magnotti, E. & Marasco, W. A. The latest animal models of ovarian cancer for novel drug discovery. Expert Opin. Drug Discov. 13, 249–257 (2018).
McIntosh, B. E. et al. Nonirradiated NOD,B6.SCID Il2rgamma-/- Kit(W41/W41) (NBSGW) mice support multilineage engraftment of human hematopoietic cells. Stem Cell Rep. 4, 171–180 (2015).
Theocharides, A. P., Rongvaux, A., Fritsch, K., Flavell, R. A. & Manz, M. G. Humanized hemato-lymphoid system mice. Haematologica 101, 5–19 (2016).
Herndler-Brandstetter, D. et al. Humanized mouse model supports development, function, and tissue residency of human natural killer cells. Proc. Natl. Acad. Sci. USA 114, E9626–E9634 (2017).
Muenst, S. et al. The immune system and cancer evasion strategies: therapeutic concepts. J. Intern. Med. 279, 541–562 (2016).
Sun, Y. et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat. Med. 18, 1359–1368 (2012).
Shiga, K. et al. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel) 7, 2443–2458 (2015).
Morton, J. J. et al. XactMice: humanizing mouse bone marrow enables microenvironment reconstitution in a patient-derived xenograft model of head and neck cancer. Oncogene 35, 290–300 (2016).
Chang, D. K. et al. Human anti-CAIX antibodies mediate immune cell inhibition of renal cell carcinoma in vitro and in a humanized mouse model in vivo. Mol. Cancer 14, 119 (2015).
Akiyama, Y. et al. The anti-tumor activity of the STAT3 inhibitor STX-0119 occurs via promotion of tumor-infiltrating lymphocyte accumulation in temozolomide-resistant glioblastoma cell line. Immunol. Lett. 190, 20–25 (2017).
Zheng, B. et al. PD-1 axis expression in musculoskeletal tumors and antitumor effect of nivolumab in osteosarcoma model of humanized mouse. J. Hematol. Oncol. 11, 16 (2018).
Hu, Z., Xia, J., Fan, W., Wargo, J. & Yang, Y.-G. Human melanoma immunotherapy using tumor antigen-specific T cells generated in humanized mice. Oncotarget 7, 6448–6459 (2016).
Tsoneva, D. et al. Humanized mice with subcutaneous human dolid tumors for immune response analysis of vaccinia virus-mediated oncolysis. Mol. Ther. Oncolytics 5, 41–61 (2017).
Jespersen, H. et al. Clinical responses to adoptive T-cell transfer can be modeled in an autologous immune-humanized mouse model. Nat. Commun. 8, 707 (2017).
Bosch, T. C. & McFall-Ngai, M. J. Metaorganisms as the new frontier. Zoology (Jena.) 114, 185–190 (2011).
Dzutsev, A., Goldszmid, R. S., Viaud, S., Zitvogel, L. & Trinchieri, G. The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur. J. Immunol. 45, 17–31 (2015).
Smith, K., McCoy, K. D. & Macpherson, A. J. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin. Immunol. 19, 59–69 (2007).
Hooper, L. V., Littman, D. R. & Macpherson, A. J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Sheflin, A. M., Whitney, A. K. & Weir, T. L. Cancer-promoting effects of microbial dysbiosis. Curr. Oncol. Rep. 16, 406 (2014).
Candela, M. et al. Inflammation and colorectal cancer, when microbiota-host mutualism breaks. World J. Gastroenterol. 20, 908–922 (2014).
Jobin, C. Colorectal cancer: looking for answers in the microbiota. Cancer Discov. 3, 384–387 (2013).
Schwabe, R. F. & Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 13, 800–812 (2013).
Sears, C. L. & Garrett, W. S. Microbes, microbiota, and colon cancer. Cell Host. Microbe 15, 317–328 (2014).
Roy, S. & Trinchieri, G. Microbiota: a key orchestrator of cancer therapy. Nat. Rev. Cancer 17, 271–285 (2017).
Arrieta, M. C., Walter, J. & Finlay, B. B. Human microbiota-associated mice: a model with challenges. Cell. Host. Microbe 19, 575–578 (2016).
Staley, C. et al. Stable engraftment of human microbiota into mice with a single oral gavage following antibiotic conditioning. Microbiome 5, 87 (2017).
Acknowledgements
C.L.: The Korean Healthcare Technology R&D project through the Korean Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (grant no. HI13C2148); the International Research & Development Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (grant no. 2015K1A4A3047851). Y.-J.C.: National Research Foundation of Korea (2017R1E1A1A01074913 and 2012R1A5A2047939). We would also like to thank Ms. Winnie Jane Cha for her help with the figures in this paper. C.L. is a distinguished Ewha Womans University Professor supported in part by the Ewha Womans University Research grant of 2017 and 2018.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, Y., Lee, S., Kim, K. et al. Studying cancer immunotherapy using patient-derived xenografts (PDXs) in humanized mice. Exp Mol Med 50, 1–9 (2018). https://doi.org/10.1038/s12276-018-0115-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-018-0115-0
This article is cited by
-
Patient-derived tumor models: a more suitable tool for pre-clinical studies in colorectal cancer
Journal of Experimental & Clinical Cancer Research (2021)
-
Development and characterization of patient-derived xenografts from non-small cell lung cancer brain metastases
Scientific Reports (2021)
-
Role of Biobanks for Cancer Research and Precision Medicine in Hepatocellular Carcinoma
Journal of Gastrointestinal Cancer (2021)
-
The tumour microenvironment shapes dendritic cell plasticity in a human organotypic melanoma culture
Nature Communications (2020)
-
Tumor xenograft animal models for esophageal squamous cell carcinoma
Journal of Biomedical Science (2018)