Abstract
Diabetic kidney disease (DKD) is the leading cause of end-stage kidney disease, and the current pharmacological treatment for DKD is limited to renin-angiotensin system (RAS) inhibitors. Adenosine is detectable in the kidney and is significantly elevated in response to cellular damage. While all 4 known subtypes of adenosine receptors, namely, A1AR, A2aAR, A2bAR, and A3AR, are expressed in the kidney, our previous study has demonstrated that a novel, orally active, species-independent, and selective A3AR antagonist, LJ-1888, ameliorates unilateral ureteral obstruction-induced tubulointerstitial fibrosis. The present study examined the protective effects of LJ-2698, which has higher affinity and selectivity for A3AR than LJ-1888, on DKD. In experiment I, dose-dependent effects of LJ-2698 were examined by orally administering 1.5, 5, or 10 mg/kg for 12 weeks to 8-week-old db/db mice. In experiment II, the effects of LJ-2698 (10 mg/kg) were compared to those of losartan (1.5 mg/kg), which is a standard treatment for patients with DKD. LJ-2698 effectively prevented kidney injuries such as albuminuria, glomerular hypertrophy, tubular injury, podocyte injury, fibrosis, inflammation, and oxidative stress in diabetic mice as much as losartan. In addition, inhibition of lipid accumulation along with increases in PGC1α, a master regulator of mitochondrial biogenesis, were demonstrated in diabetic mice treated with either LJ-2698 or losartan. These results suggest that LJ-2698, a selective A3AR antagonist, may become a novel therapeutic agent against DKD.
Similar content being viewed by others
Introduction
Diabetic kidney disease (DKD) is emerging as a worldwide public health problem and is an independent risk factor for cardiovascular morbidity and mortality1. DKD affects up to 30–40% of diabetic patients and has been recognized as a major cause of end-stage kidney disease (ESKD)2. To date, angiotensin receptor blockers (ARBs) and angiotensin converting enzyme (ACE) inhibitors are the mainstay therapeutic options for preventing the progression of DKD. However, those drugs show limitations in delaying the onset of ESKD3. It is therefore imperative to find alternative targets in halting the disease progression. Thus, the present study is focused on gaining better insight into LJ-2698, which is a new A3 adenosine receptor (A3AR) antagonist, in ameliorating DKD progression.
Adenosine is a metabolic breakdown product of adenosine triphosphate (ATP) and contributes to cytoprotection under stress, such as ischemia, hypoxia, and inflammation4. In fact, renal adenosine concentrations increase significantly in states of high renal ATP consumption, such as hypoxia and perfusion impairment5. Recent metabolomic studies have revealed a significant elevation of plasma adenosine and its derived metabolites in patients with DKD6,7. However, the role of adenosine in diabetic kidney remains elusive.
The regulation of tissue function by adenosine is mediated through activation of a G-protein coupled receptor (GPCR) family, consisting of A1, A2a, A2b, and A3 adenosine receptors (ARs)8. The A3AR is ubiquitously expressed in various tissues9. Interestingly, experimental diabetic rats10 and diabetic patient biopsies11 demonstrated that A3AR expression was up-regulated in diabetic kidneys and positively correlated with disease progression. Thus, targeting A3AR may offer a therapeutic benefit in DKD. Renoprotective effects of an A3AR antagonist have been reported in several kidney injury models, such as ischemia-reperfusion injury12, myoglobinuria-induced injury13, adriamycin-induced nephropathy14 and unilateral ureteral obstruction (UUO)-induced interstitial fibrosis15. Furthermore, a recent study reported a correlation between increased plasma concentration of adenosine and markers of renal fibrosis in diabetic rats, which were remarkably reduced by the administration of an A3AR antagonist11.
The present study investigated a newly developed A3AR antagonist, LJ-2698, which is a potent, highly selective, species-independent, and orally active agent with higher binding affinity to human A3AR than its analog, LJ-188816. In the first step, dose-dependent effects of LJ-2698 were tested (at doses of 1.5, 5, or 10 mg/kg) in db/db mice, which is a model of type 2 diabetes mellitus (T2DM). Among the 3 dosage regimens, 10 mg/kg presented significant effects in ameliorating kidney injury. Then, we compared the efficacy of LJ-2698 in ameliorating DKD with that of losartan, which is a well-established clinical drug in preventing the aggravation of DKD.
Materials and methods
Chemicals and reagents
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
Experiment I, Dose-dependent preventive effects of LJ-2698
All animal experiments were conducted according to the Institutional Animal Care and Use Committee of Ewha Laboratory Animal Genomics Center (IACUC-14-109). Eight-week-old male C57BLKS/J-db/db and age-matched control C57BLKS/J-m + /db mice (Japan SLC Inc., Hamamatsu, Japan) were housed in a room maintained at 22 ± 2℃ with a 12 h dark/12 h light cycle. To examine the preventive effects of LJ-2698 in a dose-dependent manner, LJ-2698 (1.5, 5, or 10 mg/kg) or 0.25% carboxymethyl cellulose (CMC) was administered daily to diabetic mice for 12 weeks by oral gavage. The control db/m group was administered an equal volume of CMC.
Experiment II, Renoprotective effects of LJ-2698 compared with losartan
LJ-2698 (10 mg/kg) or CMC was administered daily to control and diabetic mice for 12 weeks by oral gavage. Losartan (1.5 mg/kg) was administered in diabetic mice to compare with the effects of LJ-2698. Twelve weeks after administration, all mice were sacrificed.
Measurement of blood parameters
Before sacrificing the mice, blood samples were collected. The hemoglobin A1c (HbA1c) level was determined by a DCA2000 HbA1c reagent kit (SIEMENS Healthcare Diagnostics, Inc., Tarrytown, NY, USA). Blood glucose was measured with a glucometer (OneTouch Ultra, Johnson & Johnson Co., CA, USA). Then, blood samples were centrifuged at 3000 rpm for 15 min at 4 °C and plasma was collected. Plasma cystatin C was measured by ELISA Kits (R&D Systems, Minneapolis, MN, USA).
Measurement of urine parameters
Before sacrificing the mice, urine samples were collected in a metabolic cage for 24 h and centrifuged at 3000 rpm for 10 min. Urinary albumin excretion was measured by ELISA Kits (ALPCO, Westlake, OH, USA). The levels of urinary lipid peroxides (LPO) were measured by the thiobarbituric acid method. Urinary KIM-1 was measured by ELISA Kits (R&D Systems, Minneapolis, MN, USA).
Histology and immunohistochemistry
The right kidney was fixed with 2% paraformaldehyde-lysine-periodate (pH 7.4), dehydrated, embedded in paraffin, and sectioned. Sections were stained with periodic acid–Schiff (PAS) reagent. On each section, 15 different superficial glomeruli were randomly selected from each kidney to analyze glomerular volume and fractional mesangial area (FMA). Paraffin-embedded sections were stained using picrosirius red stain to demonstrate collagen matrix. For immunohistochemistry (IHC), anti-nephrin (PROGEN Biotechnik GmbH Inc., Heidelberg, Germany, 1:100), anti-F4/80, and anti-8-oxo-dG (Santa Cruz Biotechnology, CA, USA, 1:400) antibodies were used. Images were obtained using a Zeiss microscope equipped with an Axio Cam HRC digital camera and Axio Cam software (Carl Zeiss, Thornwood, NY, USA) and quantified by Image-Pro Plus 4.5 software (Cybernetics, Silver Spring, MD, USA).
Oxidative stress in the kidney
The extent of oxidative stress in kidney tissue was determined through measurement of LPO level utilizing the redox reaction with ferrous ion. Cayman’s Lipid Hydroperoxide Assay Kit was used for this measurement (Cayman Chemical Co, Ann Arbor, MI, USA).
Lipid accumulation
Lipid accumulation was detected by Oil Red O (ORO) staining on kidney sections. Fixed tissues were washed and incubated with 0.7% ORO for 10 min. After the samples were washed with distilled water, images were captured with a Zeiss microscope equipped with an Axio Cam HRC digital camera and Axio Cam software (Carl Zeiss, Thornwood, NY, USA).
Western blot
The protein concentration in kidney tissue homogenates was measured by the bicinchoninic acid method (Pierce Pharmaceuticals). For western blotting, protein was electrophoresed on a 10% SDS-PAGE mini-gel and transferred onto a polyvinylidene difluoride (PVDF) membrane. PVDF membranes were incubated overnight at 4°C in primary antibody, such as anti-A1AR (1:500; Abcam, Cambridge, MA, USA), anti-A2aAR (1:1000; Abcam, Cambridge, MA, USA), anti-A2bAR (1:200; Alomone Labs, Ltd. Jerusalem, Israel), anti-A3AR (1:1000; Abcam, Cambridge, MA, USA), anti-PGC1α (1:1000; Cell Signaling Technology, Danvers, MA, USA), anti-PPARα (1:1000; Abcam, Cambridge, MA, USA), and anti-COX4i1 (1:1000; Cusabio Biotech Co., Baltimore, MD, USA). Membranes were subsequently incubated with horseradish peroxidase-conjugated secondary antibody (1:1000 dilution) for 60 min at room temperature. Specific signals were detected using an enhanced chemiluminescence method (Amersham, Buckinghamshire, UK).
Real-time quantitative reverse transcription PCR (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Expression of mRNAs was measured by real-time qRT-PCR using the ABI7300 (Applied Biosystems, Carlsbad, CA, USA) with a 20 μL reaction volume consisting of cDNA transcripts, primer pairs, and SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA). Primer sequences are listed in Table 1. 18 S and β-actin were used as an internal control to normalize the genes.
Statistical analysis
Results were expressed as the mean ± SE. ANOVA was used to assess differences between multiple groups followed by Fisher post hoc analysis. The level of statistical significance was set at a p-value less than 0.05.
Results
Physical and biochemical characteristics of experimental animals
The physical and biochemical characteristics for each experimental group are presented in Tables 2 and 3 for experiments I and II, respectively. Diabetic db/db mice showed significantly higher body weight, blood glucose, HbA1c, and urine volume than non-diabetic db/m mice. Neither LJ-2698 nor losartan affected blood glucose, HbA1C, or body weight in db/db mice.
db/db Mice presents higher expression of renal A3AR than db/m mice
The expressions of all AR subtypes were initially measured in order to determine which AR subtype was significantly increased in diabetic conditions. Analysis of kidney homogenates from 20-week-old mice showed similar protein and mRNA expression levels of A1AR, A2aAR, and A2bAR between db/m and db/db mice. Only A3AR expression was increased in db/db compared to db/m mice (Fig. 1a, b).
LJ -2698 improves kidney morphology in db/db mice
To examine the effects of LJ-2698 on diabetic glomerular morphology, kidney sections were stained with PAS staining (Fig. 2a). Twenty-week-old db/db mice showed increased glomerular volume and FMA, which were effectively prevented by 12 weeks of treatment with LJ-2698 at the 3 different doses (1.5, 5, or 10 mg/kg). The reduction in glomerular volume and FMA in db/db mice was not significantly different among the 3 different doses of LJ-2698 (Fig. 2b, c).
LJ-2698 was administered to db/db mice at doses of 1.5, 5, or 10 mg/kg. CMC was administered to control db/db and db/m mice. After 12 weeks of treatment, mice were sacrificed, and the right kidney was fixed and sectioned. (a) Sections were stained with PAS reagent. The scale bar indicates 10 μm; the original magnification was ×630. (b) Glomerular volume and (c) FMA were then analyzed using Image-Pro Plus 4.5.1. Data are presented as the mean ± SE of 7–10 mice/group. *p < 0.05 vs. db/m and †p < 0.05 vs. db/db mice
LJ-2698 exhibits renoprotective effects in a dose-dependent manner in db/db mice
Next, we measured kidney injury indexes to confirm the most effective concentration of LJ-2698. Urinary albumin excretion was increased in db/db mice and was significantly reduced following 5 and 10 mg/kg treatment with LJ-2698 (Fig. 3a). Reduced nephrin and increased NGAL mRNA levels, which indicated podocyte and tubular injury in db/db mice, respectively, were effectively prevented by 10 mg/kg LJ-2698 treatment (Fig. 3b, c).
LJ-2698 was administered to db/db mice at doses of 1.5, 5, or 10 mg/kg. CMC was administered to control db/db and db/m mice. After 12 weeks of treatment, (a) urinary albumin was measured. mRNA expression levels of (b) nephrin, (c) NGAL, (d) TGF-β, (e) collagen IV, (f) fibronectin, (g) MCP-1, and (h) ICAM-1 were analyzed by qRT-PCR. (i) The urinary LPO concentration was measured by the thiobarbituric acid method. Data are presented as the mean ± SE of 7–10 mice/group. *p < 0.05 vs. db/m and †p < 0.05 vs. db/db mice
TGF-β, collagen IV, and fibronectin mRNA levels were measured as fibrosis markers. Increased TGF-β in db/db mice was inhibited only in the 10 mg/kg treatment group (Fig. 3d). Meanwhile, collagen IV and fibronectin mRNA levels were reduced by 5 and 10 mg/kg LJ-2698 treatment (Fig. 3e, f).
MCP-1 and ICAM-1 mRNA were measured as inflammation markers. The elevated MCP-1 mRNA level in db/db mice was reduced by 5 and 10 mg/kg of LJ-2698, but the ICAM-1 mRNA level was reduced only by the highest dose, 10 mg/kg of LJ-2698 (Fig. 3g, h). Additionally, urinary excretion of LPO was monitored as an index of oxidative stress. Urinary LPO was markedly increased in the db/db control group and was significantly decreased after 12-week-LJ-2698 treatment in both 5 and 10 mg/kg treatment groups (Fig. 3i).
Altogether, 10 mg/kg treatment with LJ-2698 offered sufficient effects in inhibiting the progression of kidney injury in db/db mice.
LJ-2698 prevents glomerular and tubular injury as much as losartan in db/db mice
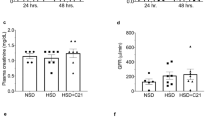
To determine whether LJ-2698 has a comparable effect with the mainstay treatment of DKD, we compared the effects of 10 mg/kg of LJ-2698 with those of 1.5 mg/kg of losartan. LJ-2698 effectively reduced urinary albumin excretion in db/db mice as much as losartan (Fig. 4a). Diabetic db/db mice showed significantly increased plasma cystatin C, which was decreased by either LJ-2698 or losartan (Fig. 4b). However, increased urinary KIM-1 excretion in db/db mice was significantly prevented only by LJ-2698 treatment (Fig. 4c).
LJ-2698 (10 mg/kg) was administered in db/db mice and age-matched control db/m mice for 12 weeks. Losartan (1.5 mg/kg) was administered in diabetic mice to compare with the effect of LJ-2698. After 12 weeks of treatment, urine and plasma were collected for analysis of (a) urinary albumin, (b) plasma cystatin C, and (c) urinary KIM-1. Paraffin-embedded kidney sections were stained with (d) PAS staining and (e) nephrin antibody. The scale bar indicates 10 μm, original magnification was ×630. Quantitative analysis of (f) glomerular volume, (g) FMA, and (h) glomerular nephrin area are depicted. Data are presented as the mean ± SE of 7–10 mice/group. *p < 0.05 vs. db/m and †p < 0.05 vs. db/db mice
The results of PAS staining (Fig. 4d) demonstrated increased glomerular volume (Fig. 4f) and FMA (Fig. 4g) in diabetic mice, which were significantly decreased by either LJ-2698 or losartan. Nephrin immuno-staining (Fig. 4e) representing podocyte integrity was significantly reduced in db/db mice, which was effectively prevented by either LJ-2698 or losartan treatment (Fig. 4h). Overall, LJ-2698 administration showed a comparable ability with losartan in ameliorating kidney injuries under diabetic conditions.
LJ-2698 inhibits kidney fibrosis in db/db mice
We further investigated the efficacy of LJ-2698 treatment in preventing the progressive accumulation of the extracellular matrix which leads to renal fibrosis. Elevated collagen IV, PAI-1, and TGF-β mRNA levels in db/db mice were significantly reduced by 12 weeks of treatment with either LJ-2698 or losartan (Fig. 5a–c). Likewise, picrosirius red staining depicting collagen accumulation in the kidney was increased in db/db mice and was significantly prevented by either LJ-2698 or losartan treatment (Fig. 5d, e).
mRNA expression levels of (a) type IV collagen, (b) PAI-1, and (c) TGF-β were determined by qRT-PCR. Representative photomicrographs of (d) picrosirius red-stained kidney sections are displayed. The scale bar indicates 50 μm; the original magnification was ×100. Quantitative analysis of (e) picrosirius red in kidney cortex is depicted. CM control mice; DM db/db diabetic mice. Data are presented as the mean ± SE of 7–10 mice/group. *p < 0.05 vs. db/m, †p < 0.05 vs. db/db, and #p < 0.05 vs. db/db + LJ-2698
LJ-2698 ameliorates kidney inflammation in db/db mice
To determine whether LJ-2698 has an anti-inflammatory effect in diabetic kidneys, we evaluated the expression of proinflammatory markers in renal tissue. Elevated mRNA levels of MCP-1 and TNF-α in db/db mice were effectively suppressed by either LJ-2698 or losartan treatment (Fig. 6a, b). Macrophage infiltration in the kidney, indicated by positive staining of F4/80, was markedly increased in db/db mice and significantly reduced by either LJ-2698 or losartan treatment (Fig. 6c, d).
After 12 weeks of LJ-2698 administration to db/db mice, mRNA expression of inflammatory cytokines in the kidney tissue was measured. (a) MCP-1 and (b) TNF-α mRNA expression was analyzed by qRT-PCR. Paraffin-embedded kidney sections were stained with (c) F4/80 antibody. The scale bar indicates 20 μm; the original magnification was ×200. Quantitative analysis of (d) F4/80 positive stained area is depicted. CM control mice; DM db/db diabetic mice. Data are presented as the mean ± SE of 7–10 mice/group. *p < 0.05 vs. db/m and †p < 0.05 vs. db/db mice
LJ-2698 prevents kidney oxidative stress in db/db mice
As oxidative stress plays a central role in the progression of kidney injury, we evaluated the state of oxidative stress in db/db mice through 8-oxo-dG immunostaining in kidney tissues and through the LPO assay in both kidney and urine samples. db/db mice presented increases in 8-oxo-dG expression and the LPO concentration in both kidney and urine samples, which were all significantly attenuated by LJ-2698 treatment. Losartan also decreased 8-oxo-dG expression and LPO concentration in the kidney but not the LPO concentration in the urine (Fig. 7a–d).
Paraffin-embedded kidney sections were stained with (a) 8-oxo-dG antibody. The scale bar indicates 20 μm; the original magnification was 200 × . Quantitative analysis of (b) 8-oxo-dG in kidney cortex is depicted. The LPO concentration was detected in both (c) kidney tissues and (d) urine samples following 12 weeks of drug treatment. CM, control mice; DM, db/db diabetic mice, Data are presented as the mean ± SE of 7–10 mice/group. *p < 0.05 vs db/m and †p < 0.05 vs db/db mice
LJ-2698 inhibits renal lipid accumulation in db/db mice
db/db mice classically represent renal lipid accumulation that contributes to the progression of kidney injury. ORO staining revealed more lipid droplets accumulated in glomeruli and tubules of db/db mice than those of db/m mice at 20 weeks old. Lipid droplets in the kidneys of db/db mice were significantly decreased by either LJ-2698 or losartan treatment (Fig. 8a, b).
(a) Renal lipids were detected by ORO staining and (b) quantified using Image-Pro Plus 4.5 software. The scale bar indicates 20 μm; the original magnification was 200 × . (c, d) Protein expression of PGC1α was detected by immunoblotting. Transcription factors interacting with PGC1α were observed, including (e) PPARα protein and (f) NRF1 mRNA. Mitochondrial transcripts were indicated by (g) TFAM mRNA. Oxidative phosphorylation was represented by (h,i) protein expression of Cox4i1 and (j) Cytb mRNA. (k) CPT1α and (l) MCAD mRNAs were analyzed to examine fatty acid β-oxidation. Data are presented as the mean ± SE of 7–10 mice/group. CM, control mice; DM, db/db diabetic mice, *p < 0.05 vs db/m, †p < 0.05 vs db/db, and #p < 0.05 vs db/db + LJ-2698
Renal PGC1α was decreased in db/db mice, which was significantly increased by either LJ-2698 or losartan treatment (Fig. 8c, d). In parallel, treated diabetic mice demonstrated increases in transcription factors, such as PPARα (Fig. 8e) and NRF1 (Fig. 8f), which interacts with PGC1α. Furthermore, db/db mice treated with either LJ-2698 or losartan exhibited elevation in i) mitochondrial transcripts indicated by TFAM (Fig. 8g), ii) oxidative phosphorylation indicated by COX4i1 (Fig. 8h, i) and Cytb (Fig. 8j), and iii) fatty acid β-oxidation presented by CPT1α and MCAD (Fig. 8k, l).
Discussion
Previous studies have demonstrated an increase in A3AR expression throughout the progression of kidney injury in both diabetic patients and experimental rat models10,11. The present study consistently showed higher renal A3AR protein expression in db/db than in db/m mice. Accordingly, highly expressed A3ARs can be a remarkable therapeutic target in halting the progression of DKD. Our present study demonstrated that 12 weeks of treatment with LJ-2698, a species-independent and orally active A3AR antagonist, improved kidney function and protected histomorphological changes in db/db mice, which is a T2DM mouse model. LJ-2698 exerted high affinity and selectivity in A3AR of both human and murine species, offering its potency to be evaluated in small-animal models and further developed as a clinical drug16. LJ-2698 prevented the progression of diabetic kidney injury in a dose-dependent manner, with the dose of 10 mg/kg showing optimal inhibition in the diabetic kidney milieu. Noticeably, the efficacy of LJ-2698 was not significantly different from losartan in ameliorating diabetic kidney injury.
Under our experimental conditions, administration of an A3AR antagonist for 12 weeks did not change renal A1AR, A2bAR, or A3AR protein expression levels in db/db mice. Interestingly, renal A2aAR protein expression was significantly increased in LJ-2698-treated mice. As A2aAR has been acknowledged to play a protective role in the diabetic kidney17,18, further study is important to elucidate the significant contributions of A2aAR elevation in protecting kidneys against diabetic injury under A3AR antagonism (Supplementary Figure 1).
In the present study, 20-week-old db/db mice exhibited higher blood glucose, HbA1C, and urinary volume than db/m mice. These biochemical characteristics were not affected by either LJ-2698 or losartan administration. Regarding the role of AR in metabolism, several reports suggested that modulation of insulin secretion and glucose homeostasis were mainly regulated via A1AR, A2aAR, and A2bAR19,20,21. A2aAR was reported to activate brown adipose tissue and recruit beige adipocytes, explaining the improvement in glucose tolerance and leaner body weight in high-fat diet (HFD)-fed mice treated with an A2aAR agonist22.
Despite the fact that LJ-2698 did not alleviate hyperglycemia, it improved kidney function in db/db mice, suggesting its direct renoprotective effects. First, we demonstrated that LJ-2698 at a dose of 10 mg/kg, but not 0.5 or 5 mg/kg, significantly decreased all parameters related to DKD progression, including glomerulosclerosis, albuminuria, inflammation, as well as podocyte and tubular injury. Then, we investigated whether 10 mg/kg LJ-2698 had a comparable efficacy to losartan, a well-established treatment for preventing the progression of DKD in T2DM23. Losartan, a drug from the ARB class, has been proved in a large clinical trial to reduce the risk of chronic kidney failure and the doubling of serum creatinine by 25–28% compared to placebo24. LJ-2698, which has a different primary target from losartan, showed a comparable reduction in albuminuria, improvement of kidney morphology, and inhibition of podocyte injury to that of losartan. Although another study showed that losartan can protect kidney tubular injury25, our study found that only LJ treatment significantly reduced urinary KIM-1, which is a sensitive marker for proximal tubular injury26. This discrepancy can be explained by different doses of losartan treatment, animal species, animal models, and parameters used for detecting tubular injury25.
Inflammatory cells and cytokines classically play a vital role in fibroblast activation in the development of kidney fibrosis, which is the final outcome of progressive kidney diseases27. Our data in mouse proximal tubular cells15 and podocyte cells (unpublished data) suggested that A3AR is activated under stimulation of either pro-fibrotic cytokines (i.e., TGF-β) or diabetic stress, such as high glucose, angiotensin-II, and palmitic acid. Inhibition of A3AR by an LJ-compound leads to a significant inhibition in fibrotic signaling in both cell types. Since A3AR may also play a role in modulating kidney hemodynamics and inflammation, the effects of A3AR antagonism in other kidney cells, such as kidney vasculatures, macrophage, and mesangial cells, should be further investigated.
In the present study, upregulated pro-inflammatory and pro-fibrotic markers in diabetic mice were significantly reduced by either LJ-2698 or losartan treatment. In regard to its mechanism of action, A3AR is a member of the GPCR family, which are coupled to Gi-mediated inhibition of adenylyl cyclase, Gq-mediated stimulation of PLC, and mitogen-activated protein kinase (MAPK) signaling pathways28. It has been well established that MAPKs, including c-Jun N-terminal kinase (JNK), extracellular signal regulated kinase (ERK) and p38 MAPK, are involved in the progression of kidney injury, which is characterized by increased extracellular matrix accumulation and epithelial to mesenchymal transition leading to kidney fibrosis29,30,31,32. Hence, these results suggest that A3AR antagonism can halt the progression of kidney fibrosis, partially through inhibition of the MAPK pathway, and remains to be elucidated in future studies.
In addition to the aforementioned results, oxidative stress markers, such as 8-oxo-dG and kidney LPO, were suppressed in both treatment groups. Interestingly, the urinary LPO concentration was significantly reduced by LJ treatment but remained unchanged in the losartan treatment group. Consistent with our findings, other studies with different kidney injury models suggested that losartan treatment could inhibit oxidative stress, as seen from reduction of parameters other than urinary LPO. Those reduced parameters were related to lipid peroxidation and activation of antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase33,34,35.
db/db mice are classically known as a renal lipotoxicity model36 induced by reduction of lipolysis via fatty acid β-oxidation. This utilization defect causes energy depletion that leads to apoptosis, dedifferentiation, eventual fibrosis and chronic kidney disease progression37. In the present study, higher renal lipid accumulation was observed in db/db than in db/m mice and was significantly decreased by either LJ-2698 or losartan treatment.
Under our experimental conditions, renal PGC1α, a master regulator of mitochondrial biogenesis, was increased in either LJ-2698- or losartan-treated mice compared to control db/db mice. It is well recognized that PGC1α interacts with transcription factors, such as ERRα, PPAR, NRF1, and NRF2, whose target genes increase mitochondrial biogenesis, oxidative phosphorylation, and fatty acid β-oxidation38,39. In parallel, elevation of PPARα and NRF1 was observed in diabetic mice treated with either LJ-2698 or losartan. The increased PGC1α protein level indirectly implies the improvement of mitochondrial biogenesis, oxidative phosphorylation, and fatty acid β-oxidation, as shown by the elevation of TFAM; COX4i1 and Cytb; and CPT1α and MCAD, respectively.
In summary, we are the first to show that LJ-2698, a highly selective and species-independent A3AR antagonist, ameliorated diabetic kidney injury in db/db mice. LJ-2698 ameliorated the progression of diabetic kidney injury to the same extent as losartan. Oxidative stress, inflammation, lipid accumulation, albuminuria, glomerulosclerosis, and tubulointerstitial fibrosis were all aggravated in db/db mice and significantly reversed by LJ-2698 treatment. Thus, we suggest that LJ-2698 can be a new treatment option in preventing diabetic kidney progression.
For further insight into the efficacy of LJ-2698, therapeutic effects of delayed treatment with LJ-2698 in DKD should be investigated. Potential effects of LJ-2698 in adipose and cardiac tissues, which are related to modulation of A3AR antagonism on metabolism and long-term complication in DKD, should be elucidated. Finally, since LJ-2698 and losartan have different primary targets in protecting the kidneys, it is also imperative to investigate the renoprotective effects of the combination therapy of both drugs in DKD.
References
Sarnak, M. J. et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 42, 1050–1065 (2003).
Gross, J. L. et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28, 176–188 (2005).
Vilayur, E. & Harris, D. C. Emerging therapies for chronic kidney disease: what is their role? Nat. Rev. Nephrol. 5, 375–383 (2009).
Vallon, V., Muhlbauer, B. & Osswald, H. Adenosine and kidney function. Physiol. Rev. 86, 901–940 (2006).
Rabadi, M. M. & Lee, H. T. Adenosine receptors and renal ischaemia reperfusion injury. Acta Physiol. 213, 222–231 (2015).
Xia, J. F. et al. Correlations of six related purine metabolites and diabetic nephropathy in Chinese type 2 diabetic patients. Clin. Biochem. 42, 215–220 (2009).
Xia, J. F. et al. Ultraviolet and tandem mass spectrometry for simultaneous quantification of 21 pivotal metabolites in plasma from patients with diabetic nephropathy. J. Chromatogr. 877, 1930–1936 (2009).
Bauerle, J. D., Grenz, A., Kim, J. H., Lee, H. T. & Eltzschig, H. K. Adenosine generation and signaling during acute kidney injury. J. Am. Soc. Nephrol. 22, 14–20 (2011).
Borea, P. A. et al. The A3 adenosine receptor: history and perspectives. Pharmacol. Rev. 67, 74–102 (2015).
Pawelczyk, T., Grden, M., Rzepko, R., Sakowicz, M. & Szutowicz, A. Region-specific alterations of adenosine receptors expression level in kidney of diabetic rat. Am. J. Pathol. 167, 315–325 (2005).
Kretschmar, C. et al. Reduced adenosine uptake and Its contribution to signaling that mediates profibrotic activation in renal tubular epithelial cells: Implication in diabetic nephropathy. PLoS ONE 11, e0147430 (2016).
Lee, H. T. & Emala, C. W. Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A1 and A3 receptors. Am. J. Physiol. 278, F380–F387 (2000).
Lee, H. T., Ota-Setlik, A., Xu, H., D’ Agati V. D., Jacobson M. A. & Emala C. W. A3 adenosine receptor knockout mice are protected against ischemia-and myoglobinuria-induced renal failure. Am. J. Physiol. Ren. Physiol. 284, F267–F273 (2003).
Min, H. S. et al. Renoprotective effects of a highly selective A3 adenosine receptor antagonist in a mouse model of adriamycin-induced nephropathy. J. Korean Med. Sci. 31, 1403–1412 (2016).
Lee, J. et al. The selective A3AR antagonist LJ-1888 ameliorates UUO-induced tubulointerstitial fibrosis. Am. J. Pathol. 183, 1488–1497 (2013).
Jeong, L. S. et al. Discovery of a new nucleoside template for human A3 adenosine receptor ligands: D-4’-thioadenosine derivatives without 4’-hydroxymethyl group as highly potent and selective antagonists. J. Med. Chem. 50, 3159–3162 (2007).
Awad, A. S. et al. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 290, F828–F837 (2006).
Persson, P. et al. Adenosine A2 a receptor stimulation prevents proteinuria in diabetic rats by promoting an anti-inflammatory phenotype without affecting oxidative stress. Acta Physiol. 214, 311–318 (2015).
Antonioli, L., Blandizzi, C., Csoka, B., Pacher, P. & Hasko, G. Adenosine signalling in diabetes mellitus--pathophysiology and therapeutic considerations. Nat. Rev. Endocrinol. 11, 228–241 (2015).
Faulhaber-Walter, R. et al. Impaired glucose tolerance in the absence of adenosine A1 receptor signaling. Diabetes 60, 2578–2587 (2011).
Csóka, B. et al. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes Care 63, 850–866 (2014).
Gnad, T. et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516, 395–399 (2014).
Hall, P. M. Prevention of progression in diabetic nephropathy. Diabetes Spectr. 19, 18–24 (2006).
Brenner, B. M. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. New Engl. J. Med. 345, 861–869 (2001).
He, P., Li, D. & Zhang, B. Losartan attenuates renal interstitial fibrosis and tubular cell apoptosis in a rat model of obstructive nephropathy. Mol. Med. Rep. 10, 638–644 (2014).
Han, W. K., Bailly, V., Abichandani, R., Thadhani, R. & Bonventre, J. V. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 62, 237–244 (2002).
Kanasaki, K., Taduri, G. & Koya, D. Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Front. Endocrinol. 4, 7 (2013).
Hasko, G., Linden, J., Cronstein, B. & Pacher, P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug. Discov. 7, 759–770 (2008).
Liu, Q. et al. A crosstalk between the Smad and JNK signaling in the TGF-beta-induced epithelial-mesenchymal transition in rat peritoneal mesothelial cells. PLoS ONE 7, e32009 (2012).
Stambe, C. The role of p38 mitogen-activated protein kinase activation in renal fibrosis. J. Am. Soc. Nephrol. 15, 370–379 (2004).
Ma, F. Y. et al. A pathogenic role for c-Jun amino-terminal kinase signaling in renal fibrosis and tubular cell apoptosis. J. Am. Soc. Nephrol. 18, 472–484 (2007).
Pat, B. et al. Activation of ERK in renal fibrosis after unilateral ureteral obstruction: modulation by antioxidants. Kidney Int. 67, 931–943 (2005).
Arozal, W., Watanabe, K. & Veeraveedu, P. T. Effects of angiotensin receptor blocker on oxidative stress and cardio-renal function in streptozotocin-Induced diabetic rats. Biol. Pharm. Bull. 32, 1411–1416 (2009).
Ivanov, M. et al. Losartan improved antioxidant defense, renal function and structure of postischemic hypertensive kidney. PLoS ONE 9, e96353 (2014).
Karanovic, D. et al. Effects of single and combined losartan and tempol treatments on oxidative stress, kidney structure and function in spontaneously hypertensive rats with early course of proteinuric nephropathy. PLoS ONE 11, e0161706 (2016).
Wang, Z. et al. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes 54, 2328–2335 (2005).
Stadler, K., Goldberg, I. J. & Susztak, K. The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr. Diab. Rep. 15, 40 (2015).
Galvan, D. L., Green, N. H. & Danesh, F. R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 92, 1051–1057 (2017).
Lin, J., Handschin, C. & Spiegelman, B. M. Metabolic control through the PGC-1 family of transcription coactivators. Cell. Metab. 1, 361–370 (2005).
Acknowledgements
This work was supported by NRF-2014R1A2A1A11050945 from the National Research Foundation of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. If you remix, transform, or build upon this article or a part thereof, you must distribute your contributions under the same license as the original. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/.
About this article
Cite this article
Dorotea, D., Cho, A., Lee, G. et al. Orally active, species-independent novel A3 adenosine receptor antagonist protects against kidney injury in db/db mice. Exp Mol Med 50, 1–14 (2018). https://doi.org/10.1038/s12276-018-0053-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-018-0053-x
This article is cited by
-
PGC1-α in diabetic kidney disease: unraveling renoprotection and molecular mechanisms
Molecular Biology Reports (2024)