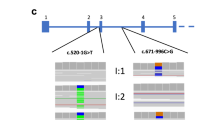
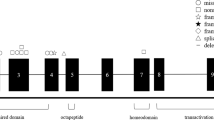
Abstract
Nephronophthisis is an autosomal-recessive kidney disease that is caused by abnormalities in primary cilia. Nephronophthisis-related ciliopathies (NPHP-RCs) are a common cause of end-stage kidney disease (ESKD) in children and adolescents. NPHP-RCs are often accompanied by extrarenal manifestations, including intellectual disability, retinitis pigmentosa, or polydactyly. Although more than 100 causative genes have been identified, its diagnosis is difficult because the clinical features of each mutation often overlap. From September 2010 to August 2021, we performed genetic analysis, including next-generation sequencing (NGS), in 574 probands with kidney dysfunction and retrospectively studied cases genetically diagnosed with NPHP-RCs.
Results
We detected mutations related to NPHP-RCs in 93 patients from 83 families. Members of 60 families were diagnosed using NGS, and the mutations and the corresponding number of families are as follows: NPHP1 (24), NPHP3 (10), OFD1 (7), WDR35 (5), SDCCAG8 (4), BBS10 (3), TMEM67 (3), WDR19 (3), BBS1 (2), BBS2 (2), IFT122 (2), IFT140 (2), IQCB1 (2), MKKS (2), SCLT1 (2), TTC21B (2), ALMS1 (1), ANKS6 (1), BBS4 (1), BBS12 (1), CC2D2A (1), DYNC2H1 (1), IFT172 (1), and MAPKBP1 (1). A total of 39 cases (41.9%) progressed to ESKD at the time of genetic analysis, whereas 58 cases (62.3%) showed extrarenal manifestations, the most common being developmental delay, intellectual disability, and autism spectrum disorder in 44 patients. Comprehensive genetic analysis using NGS is useful for diagnosing patients with NPHP-RCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Wolf MT, Hildebrandt F. Nephronophthisis. Pediatr Nephrol. 2011;26:181–94.
Hildebrandt F, Otto E. Molecular genetics of nephronophthisis and medullary cystic kidney disease. J Am Soc Nephrol. 2000;11:1753–61.
Hildebrandt F, Waldherr R, Kutt R, Brandis M. The nephronophthisis complex: clinical and genetic aspects. Clin Investig. 1992;70:802–8.
Simms RJ, Hynes AM, Eley L, Sayer JA. Nephronophthisis: a genetically diverse ciliopathy. Int J Nephrol. 2011;2011:527137.
Srivastava S, Sayer JA. Nephronophthisis. J Pediatr Genet. 2014;3:103–14.
Stokman M, Lilien M, Knoers N. Nephronophthisis. In: Adam MP, Ardinger HH, Pagon RA, et al. ed. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 2016; 1993–2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK368475/
Halbritter J, Porath JD, Diaz KA, Braun DA, Kohl S, Chaki M, et al. Identification of 99 novel mutations in a worldwide cohort of 1,056 patients with a nephronophthisis-related ciliopathy. Hum Genet. 2013;132:865–84.
Braun DA, Schueler M, Halbritter J, Gee HY, Porath JD, Lawson JA, et al. Whole exome sequencing identifies causative mutations in the majority of consanguineous or familial cases with childhood-onset increased renal echogenicity. Kidney Int. 2016;89:468–75.
Gee HY, Otto EA, Hurd TW, Ashraf S, Chaki M, Cluckey A, et al. Whole-exome resequencing distinguishes cystic kidney diseases from phenocopies in renal ciliopathies. Kidney Int. 2014;85:880–7.
Kang HG, Lee HK, Ahn YH, Joung JG, Nam J, Kim NDK, et al. Targeted exome sequencing resolves allelic and the genetic heterogeneity in the genetic diagnosis of nephronophthisis-related ciliopathy. Exp Mol Med. 2016;48:e251.
Schueler M, Halbritter J, Phelps IG, Braun DA, Otto EA, Porath JD, et al. Large-scale targeted sequencing comparison highlights extreme genetic heterogeneity in nephronophthisis-related ciliopathies. J Med Genet. 2016;53:208–14.
Blowey DL, Querfeld U, Geary D, Warady BA, Alon U. Ultrasound findings in juvenile nephronophthisis. Pediatr Nephrol. 1996;10:22–24.
König J, Kranz B, König S, Schlingmann KP, Titieni A, Tönshoff B, et al. Phenotypic spectrum of children with nephronophthisis and related ciliopathies. Clin J Am Soc Nephrol. 2017;12:1974–83.
Stokman MF, van der Zwaag B, van de Kar NCA, van Haelst MM, van Eerde AM, van der Heijden JW, et al. Clinical and genetic analyses of a Dutch cohort of 40 patients with a nephronophthisis-related ciliopathy. Pediatr Nephrol. 2018;33:1701–12.
Srivastava S, Molinari E, Raman S, Sayer JA. Many genes-one disease? genetics of nephronophthisis (NPHP) and NPHP-associated disorders. Front Pediatr. 2017;5:287.
Wolf MT. Nephronophthisis and related syndromes. Curr Opin Pediatr. 2015;27:201–11.
Hildebrandt F, Rensing C, Betz RC, Sommer U, Brinbaum S, Imm A, et al. Establishing an algorithm for molecular genetic diagnostics in 127 families with juvenile nephronophthisis. Kidney Int. 2001;59:434–45.
Snoek R, Van Setten J, Keating BJ, Israni AK, Jacobsen PA, Oetting WS, et al. NPHP1 (Nephrocystin-1) gene deletions cause adult-onset ESRD. J Am Soc Nephrol. 2018;29:1772–9.
Macferran KM, Buchmann RF, Ramakrishnaiah R, Griebel ML, Sanger WG, Saronwala A, et al. Pontine tegmental cap dysplasia with a 2q13 microdeletion involving the NPHP1 gene: insights into malformations of the mid-hindbrain. Semin Pediatr Neurol. 2010;17:69–74.
Inaguma Y, Kaito H, Morisada N, Iijima K, Tanaka R. Renal-hepatic-pancreatic dysplasia-1 diagnosed on comprehensive gene analysis. Pediatr Int. 2019;61:210–2.
Sakakibara N, Morisada N, Nozu K, Nagatani K, Ohta T, Shimizu J, et al. Clinical spectrum of male patients with OFD1 mutations. J Hum Genet. 2019;64:3–9.
Acknowledgements
We thank all the patients, their social guardians, and primary doctors. We are grateful to Ms. Yoshimi Nozu, Ms. Yuko Noguchi, and Ms. Tetsuko Yamanouchi (Kobe University) for their excellent technical assistance. The following doctors provided patient samples for the study: Takayuki Okamoto (Hokkaido University), Tomomi Aizawa (Hirosaki University), Akira Ashida (Osaka Medical University), Shunsuke Goto, Hideki Fujii (Kobe University Graduate School of Medicine), Kouki Tomari (Tokyo Metropolitan Children’s Hospital), Satoshi Hibino (Aichi Children’s Health and Medical Center), Takashi Iijima, Yoshifumi Ubara (Toranomon Hospital), Marie Ito, Shunsuke Sakurai (Showa University Fujigaoka Hospital), Hidefumi Kishikawa (Hyogo Prefectural Nishinomiya Hospital), Koichi Kamei, Mika Okutsu, Ryutaro Suzuki, Mai Sato, Toru Kanamori (National Center for Child Health and Development), Rie Kawakita, Rika Fujimaru, Ichiro Kuki (Osaka City General Hospital), Takayuki Miyai (Okayama University), Kazuetsu Mori (Seirei Sakura Citizen Hospital), Masayuki Nakazawa (Sasebo Chuo Hospital), Ayumi Nogi (Ome Municipal Hospital), Katsusuke Yamamoto (Osaka Women’s and Children’s Hospital), Ken Saida (Yokohama City University), Takayuki Shibano (Hyogo College of Medicine), Junya Shimizu (NHO Okayama Medical Center), Ryojiro Tanaka (Hyogo Prefectural Kobe Children’s Hospital), Emi Utsuno (Chiba University), Takuzo Wada (Kinan Hospital), Masato Yasui (Fukuyama City Hospital), Shuichiro Fujinaga, Yoshitaka Watanabe, Shota Endo (Saitama Prefectural Children’s Medical Center), Tetsuji Inagaki (Miyagi Children’s Hospital), Masaki Takahashi (Tokyo Metropolitan Bokutoh Hospital), Tadashi Sofue (Kagawa University), Takeshi Yamada, Keisuke Nagasaki, Hiroya Hasegawa (Niigata University), Ayako Saito (Ibaraki Children’s Hopital), Ichiro Hada, Eriko Tanaka (Kyorin University), Yoshinobu Nagaoka (Sapporo Medical University), Tomo Suzuki (Kameda Medical Center), Takashi Uzu (Nippon Life Hospital), Masayuki Iwano (Fukui University), Toshihiko Shirakawa (Nagasaki University), Tomoko Nakamura (Odawara Municipal Hospital), Ryoichi Kitagata (Hamamatsu University School of Medicine), Daisuke Ichikawa (St. Marianna University School of Medicine), Masaki Yamamoto (Seirei Hamamatsu Hospital), Hisatsugu Takahara (Juntendo University Urayasu Hospital), Takashi Ohmae (Nara Prefectural Medical University), Kenichiro Miura (Tokyo Women’s Medical University).
Funding
This work was supported by the Health Labor Sciences Research Grant for the Research on Measures for Intractable Diseases (H24-nanchi-ippan-041 to K.I.; H29-nanchi-ippan-039 to NM) and the Japan Society for the Promotion of Science (KAKENHI Grant Numbers JP15K09261 and 18K08243 to NM)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
MM reports the following: Current Employer: Kobe Gakuin University which is financially supported by KNC Laboratories Co. Ltd. Inc. Japan. Consultancy Agreements: JCR Pharma Co., Ltd., Japan; Daiichi Sankyo Co., Ltd., Japan. Research Funding: Daiichi Sankyo Co., Ltd., Japan.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sakakibara, N., Nozu, K., Yamamura, T. et al. Comprehensive genetic analysis using next-generation sequencing for the diagnosis of nephronophthisis-related ciliopathies in the Japanese population. J Hum Genet 67, 427–440 (2022). https://doi.org/10.1038/s10038-022-01020-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-022-01020-5